Fig. 5.
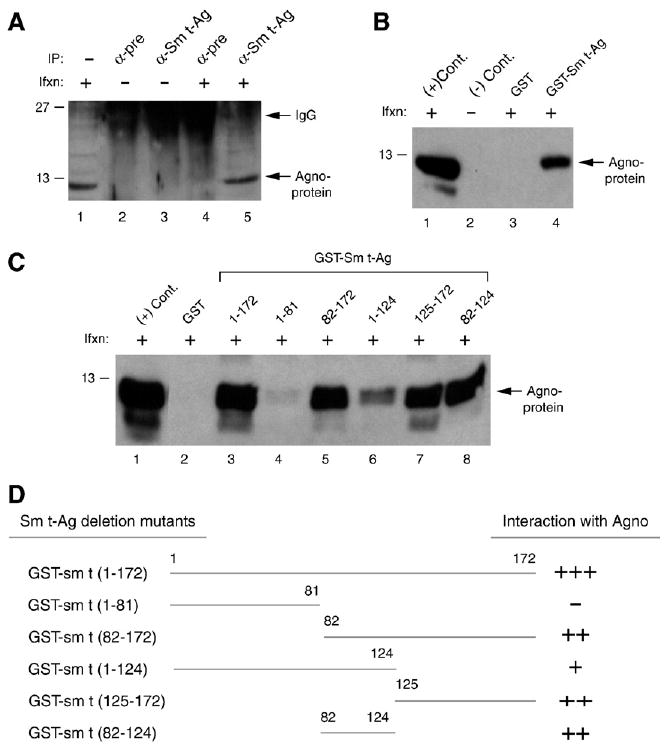
The C-terminal portion of Sm t-Ag is important for agnoprotein interaction. (A) Agnoprotein coimmunoprecipitates with Sm t-Ag. Whole cell extracts (500 μg) prepared from SVG-A cells infected (Ifxn) or uninfected with JCV Mad-1 were subjected to immunoprecipitation (IP) using α-pre rabbit and α-Sm t-Ag antibody and analyzed by Western blotting using α-agno antibody. In lane 1, whole cell extracts from infected cells were loaded as a positive control. (B) GST pulldown assay. Whole cell extracts, prepared from SVG-A cells, infected with JCV Mad-1 were incubated with either GST or GST–Sm t-Ag, immobilized on Glutathione–Sepharose beads. Bound proteins were analyzed by Western blotting using an α-agno antibody as described for Fig. 2. In lane 1, whole cell extract (20 μg) from infected cells was loaded as a positive (+) control. In lane 2, whole cell extract (20 μg) from uninfected cells was loaded as a negative (−) control. (C) The agnoprotein interaction domain of Sm t-Ag maps to the C-terminal portion of the protein. GST pulldown assay was carried out as described for panel B. (D) Summary of the results from in vitro mapping assays as described for Fig. 4C.
