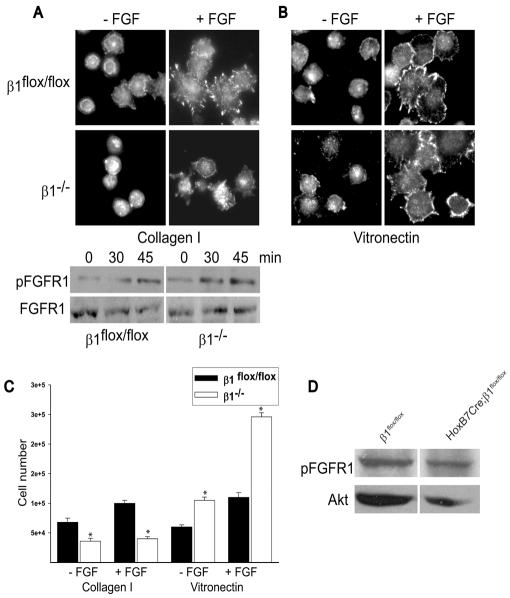
Fig. 7.
FGFR1 is localized to the cell membrane and is equally phosphorylated in β1flox/flox and β1-/- cells. (A,B) β1flox/flox and β1-/- CD cells were allowed to adhere for 30 minutes to collagen I (A) or vitronectin (B) (both 10 μg/ml), after which they were incubated with or without FGF2 (FGF) (10 ng/ml) for 1 hour and then stained with an anti-pFGFR1 antibody. The lower panel in A is an immunoblot showing the levels of pFGFR1 and total FGFR1 in β1flox/flox and β1-/- CD that were allowed to adhere to collagen I for 30 minutes and were then treated with FGF2 for the times indicated. (C) In six-well plates coated with collagen I or vitronectin (10 μg/ml) with or without FGF2 (10 ng/ml), 3×105 β1flox/flox and β1-/- CD cells were grown. Forty-eight hours later the cells were trypsinized and counted. Values are the mean ± s.d. of three different experiments. Asterisks indicate statistically significant differences (P<0.05) between β1flox/flox and β1-/- CD cells. (D) Medullas of P1 β1flox/flox and HoxB7Cre;β1flox/flox mice were lysed and 20 μg of total cell lysates were immunoblotted for levels of pFGFR1. Equal protein loading was verified by incubating the blots with anti-Akt antibodies.