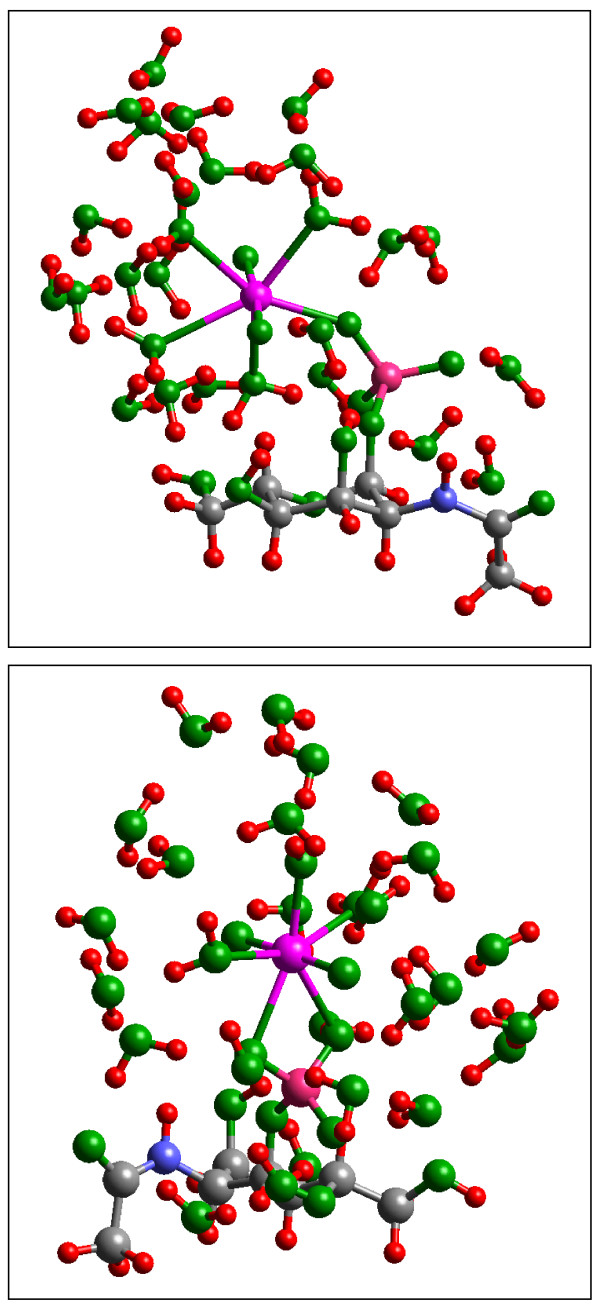
Figure 11.
(a) The monodentate UO2-GlcNPO4 complex has a calculated potential energy +70 kJ/mol higher than the corresponding (b) bidentate complex, but the former has interatomic distances in closer agreement with EXAFS data on uranyl adsorbed onto bacterial surfaces [47]. These two facts suggest that protonation of the phosphoryl group at low pH stabilizes the monodentate configuration and that the bidentate configuration should be more stable at circumneutral pH.