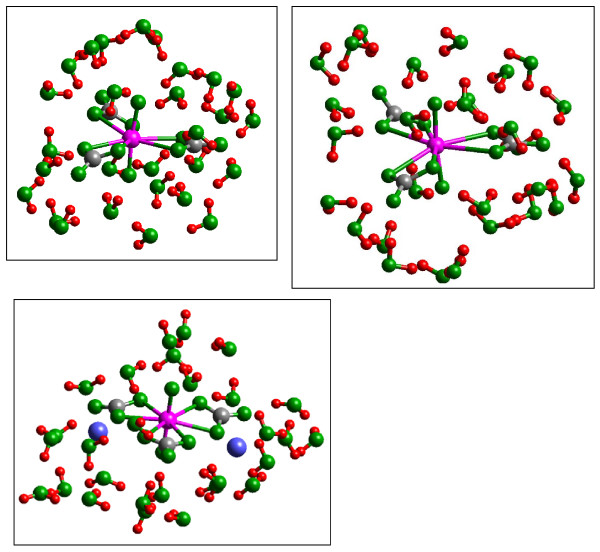
Figure 2.
Model structures of the aqueous species (a) UO2(CO3)34-•28(H2O), (b) UO2(CO3)35-•28(H2O) and (c) Ca2UO2(CO3)3•28(H2O) in-plane configuration. (a) Strong H-bonding to the carbonate groups weakens the U-carbonate bonding by approximately 0.1 Å to bring the calculated value in better agreement with observation (Table 1; [42]). H-bonds to the O atoms of the UO22+ group are relatively weak. Reduction of the U atom to U(V) causes a slight twisting of the carbonate ligands as proposed by Docrat et al. [43]. H-bonding to the uranyl O atoms becomes relatively stronger compared to the analogous U(VI) complex. (c) Addition of Ca2+ ions to charge-balance this model aqueous species results in a configuration close to the observed crystal structure of the mineral liebigite [45].