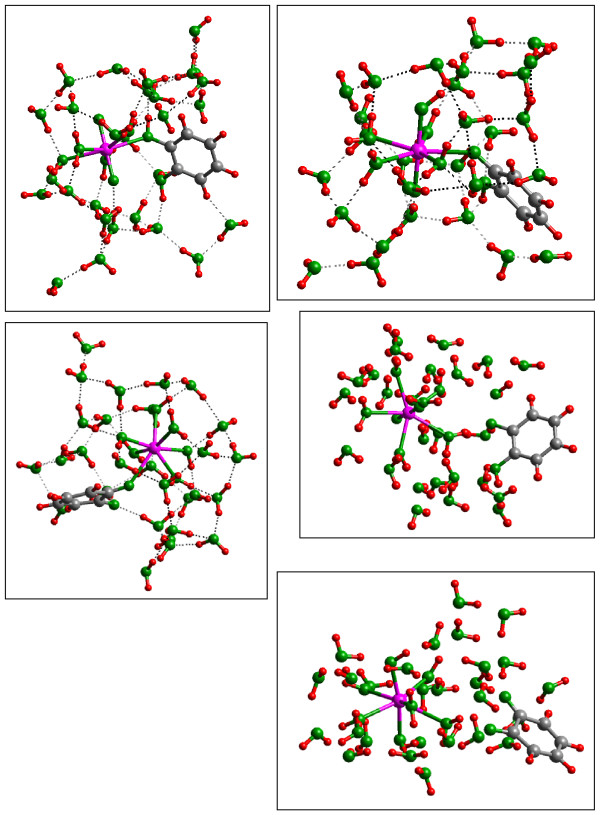
Figure 9.
(a) [UO2(OH2)4]2+-H2Cat•28(H2O) singlet (=UO2-H2Cat), (b) [UO(OH)(OH2)4]+-HCat•28(H2 O) triplet (=UO2 -HCat), (c) [U(OH)2 (OH2)4]-Cat•28(H2O) quintet (=U(OH)2-Cat), (d) outer-sphere [UO2(OH2)4]2+-H2 Cat•28(H2O) (=UO2-H2Cat OS), (e) U(OH)2(OH2)4(C6H4O2)•28(H2O) (=U(OH)2-Quin). Note the single bond to catechol in (a) and the H transfer to the axial uranyl O atoms in (b) concomitant with the changing electronic state and uranyl reduction (Table 3). The most stable state calculated is (e) where the uranyl has been reduced and a quinone has generated. This result is consistent with the experimental observations of [100] presuming that the U(IV) produced in this reaction was re-oxidized by O2 in these aerobic experiments.