Figure 2.
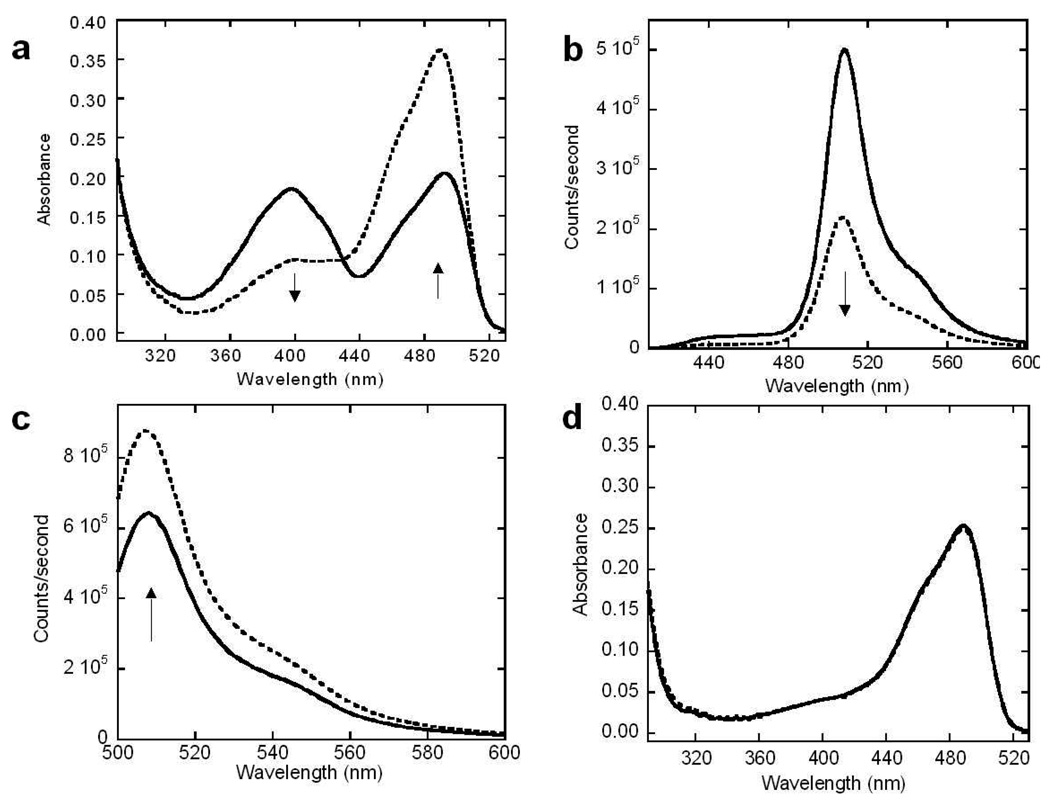
Optical change and kinetic studies of trypsin sensors. Absorption spectra of EGFP-T1 (a) were measured after trypsin digestion at various times from 0 min (solid line) to 120 min (dashed line) in trypsin digestion buffer (10 mM Tris, 20 mM CaCl2, at pH 7.4). Absorbance of EGFP-T1 decreased at 397 nm and increased at 491 nm following trypsin digestion at different digestion time intervals. The fluorescence spectra of EGFP-T1 with excitation at 398 nm (b) and excitation at 490 nm (c) were measured following trypsin digestion from 0 min (solid line) to 120 min (dashed line) in trypsin digestion buffer, respectively. Fluorescence of EGFP-T1 decreased when excited at 397 nm and increased when excited at 491 nm following trypsin digestion at different digestion time intervals. There is not an absorption spectrum change of EGFP-T2 (d) following a trypsin digestion.