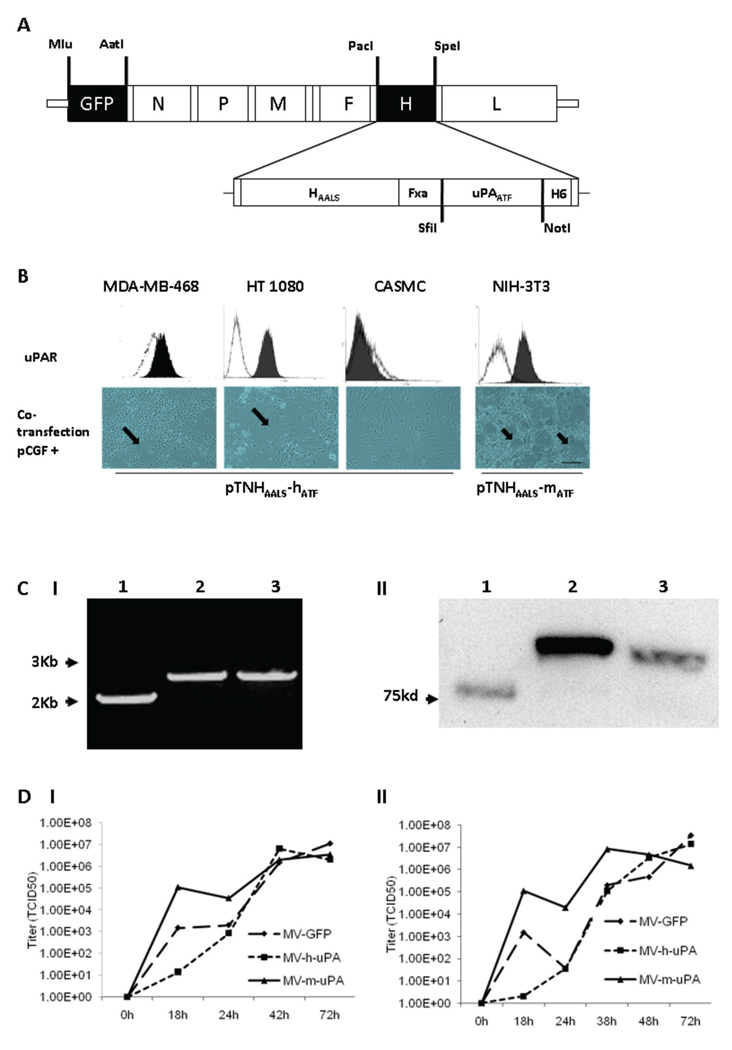
Figure 1. Rescue and in vitro characterization of recombinant oncolytic measles viruses fully retargeted against human and murine uPAR.
(A) Schematic representation of the recombinant retargeted measles virus genome. The amino terminal fragment (ATF) of human or murine uPA, flanked by the SfiI/NotI restriction sites was displayed as a C-terminal extension of HAALS, a measles virus H glycoprotein with 4 residue mutations (AALS: Y481A, R533A, S548L, F549S) that ablate its ability to bind CD46 and SLAM (32). (B) Functional fusion formation assay of chimeric MV-H glycoproteins. After assessing expression of human (MDA-MB-468, HT-1080 and CASMC cells) and murine (NIH-3T3) uPAR by FACS (upper panel), cells were cotransfected with pCGF, a mammalian expression vector encoding MV-F (fusogenic) glycoprotein and either pTNHAALS-h-ATF (MDA-MB-468, HT1080 and CASMC) or pTNHAALS-m-ATF (NIH-3T3). Cell fusion was observed in the human (MDA-MB-231, HT1080) and murine (NIH-3T3) tumorigenic cells (arrows), but not in non-cancer cells (CASMC) 48 hours after cotransfection. Scale bar = 500 µm. After confirming functional activity of HAALS-ATF (human and murine), the chimeric glycoproteins were cloned individually into the PacI/SpeI sites of the measles virus genome (Fig. 1A). The human and murine retargeted viruses were named MV-h-uPA and MV-m-uPA, respectively. The enhanced green fluorescent protein (eGFP) gene was inserted into the Mlu/AatI site before the N gene. Fxa= factor Xa cleavage site (IEGR). H6= six-histidine peptide. N= nucleocapsid, P= phosphoprotein. M= matrix; F= fusion. H= hemagglutinin; L= polymerase gene. (C). I, RT-PCR analysis of MV-H glycoproteins in uPAR retargeted MVs (Left panel). Lane 1, MV-GFP; lane 2, chimeric MV-m-uPA; lane 3, MV- h-uPA. II, Immunoblot analysis of viral H protein using anti-H antibody. Equal titers of each virus were loaded. Lane 1, unmodified control H protein with mobility at 75 kDa; lane 2, chimeric MV-m-uPA; lane 3, MV- h-uPA. (D). In vitro viral propagation analysis of MV-h-uPA and MV-m-uPA by the one step growth curve in Vero-αHis cells. Titers (TCID50) of retargeted viruses were comparable to the unablated control virus (MV-GFP). I, Cell associated virus; II, cell released virus.