Figure 1.
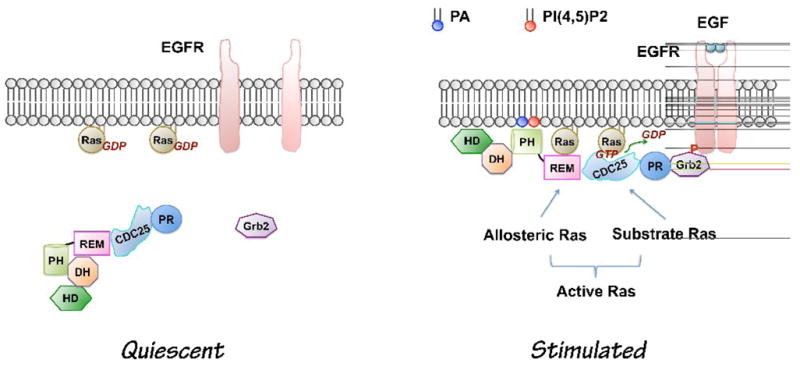
Current model of growth factor-induced Sos activation (Modified based on [20]). In quiescent cells, PLD activity is low. The DH-PH domains block the allosteric binding site for Ras. Upon growth factor stimulation, Sos is recruited to the plasma membrane through at least two independent sites: C-terminal Grb2-binding site and its lipid PA- and PI(4,5)P2 -binding PH domain. Interaction of the PH domain with phospholipids leads to conformational changes allowing binding of allosteric Ras, which lead to the maximal activation of Sos. PA level is increased by receptor activation of PLD2 [17]. The role of PA from other sources in growth factor-induced Sos activation has not been tested yet. CDC25, CDC25-homology domain, DH, Dbl homology domain; EGF, Epidermal growth factor; EGFR, Epidermal growth factor receptor; HD, Histone-like domain; PH, pleckstrin homology domain; PR, Proline-rich motif; REM, Ras exchanger motif; Phosphatidic acid, PA; Phosphatidylinositol-4,5-bisphosphate; PI(4,5)P2.
