Abstract
OBJECTIVES
To identify factors associated with sustained benzodiazepine use in older adults
DESIGN
12-year cohort study
SETTING
Community-based epidemiologic survey.
PARTICIPANTS
1,342 individuals aged 65+ years
MEASUREMENTS
Demographics, medication use, depressive symptoms, sleep complaints, alcohol use, and smoking, assessed at two-year intervals; descriptive analysis to characterize benzodiazepine users and identify factors associated with sustained benzodiazepine use (use at two consecutive waves); longitudinal lag-time analysis to determine characteristics that predicted sustained use.
RESULTS
Initially, 5.5 % of men and 9.8 % of women were using benzodiazepines. Users were significantly more likely than non-users to be women, less educated, report more depressive and anxiety symptoms, use more prescription medications, have lower self-rated health, have difficulty maintaining sleep, and less likely to consume alcohol. Approximately 50%, 44%, and 25% of these users aged 65-74. 75-84, and 85+ were sustained users at follow-up. Being female, using two or more non-benzodiazepine prescription medications, and smoking were independently associated with subsequent sustained benzodiazepine use.
CONCLUSION
At the population level, women, smokers, and users of at least two prescription drugs have elevated probabilities of sustaining benzodiazepine use once started. This information can facilitate risk assessment and counseling of older adults before prescribing benzodiazepines.
Keywords: aging, anxiolytics, MoVIES
INTRODUCTION
Benzodiazepines are a class of compounds that increase activity at the gamma-aminobutyric acid-A (GABAA) receptor activity via binding at the site of the benzodiazepine receptor. Most are absorbed fully from the gastrointestinal tract and are hepatically metabolized. Therapeutic indications include treatment of insomnia, generalized anxiety disorder, panic disorder, and social phobia. They are also commonly used for acute agitation and as adjunctive treatment for mania. Further uses include skeletal muscle relaxation and adjunctive treatment for seizure disorders. Quickly absorbed benzodiazepines, such as lorazepam, diazepam, and alprazolam, are often used for episodes of acute anxiety or for insomnia. The half-life of these drugs vary from less than five hours (triazolam) to more than 200 hours in the presence of long-acting metabolites (diazepam).1
Older adults are among the largest users of benzodiazepines, typically prescribed for sleep-related difficulties and anxiety.2 Generally, benzodiazepines are indicated for short-term symptom relief and have considerable potential efficacy when used in this manner.3 However, the use of benzodiazepines is not without its risks.4 Falls and cognitive impairment are the most widely reported adverse effects with short-term use.5 Cognitive decline, an increased risk of falls, and development of dependence have been shown with longer-term benzodiazepine use.6-8 Although all long-term benzodiazepine use must begin as short-term use, not all short-term use leads to long-term use. Therefore, clinicians’ ability to assess older adults for risk of long term benzodiazepine use would be enhanced by knowledge of patients’ characteristics predictive of sustained and chronic use.
Prior studies, typically cross-sectional, have found older age, female gender, and a larger overall number of prescription medications to be associated with the prescription of benzodiazepines.9, 10 However, cross-sectional studies do not allow models to test covariate effects on long-term outcomes. Other epidemiologic studies have focused on predictors of benzodiazepines therapy changes, such as dose changes or therapeutic switching, and factors associated with either short or long term use.11, 12 In the context of a population-based cohort study, we set out to identify factors that might be associated with sustained benzodiazepine use.
METHODS
The Monongahela Valley Independent Elders Survey (MoVIES) was a prospective epidemiologic study amongst older adults conducted from 1987-2002 in southwestern Pennsylvania. Details of sampling and recruitment have been published previously.13 In brief, we recruited an original cohort of 1,681 individuals who in 1987 were aged 65 and older and living in the mid-Monongahela Valley, a largely rural, post-industrial area of relatively low socioeconomic status. At the recruitment wave (Wave 1), the original cohort had a mean (SD) age of 72.1 (5.9) years, was approximately 55% female, 97% white (reflecting the elderly base population of the rural mid-Monongahela Valley), and had a median educational level of high school graduate. We followed the cohort with repeated biennial assessments until 2002.
At each data collection wave, participants provided current prescription drug data including medication being taken either regularly or only as needed. Data collected regarding over-the-counter drug use are not reported here, as benzodiazepines are legally available only by prescription. Drug names were confirmed by direct inspection of the medication bottles in the home. The total number of prescription drugs that participants reported taking regularly was used in these analyses as a measure of overall morbidity (including psychiatric morbidity) at each wave.14 To avoid collinearity between benzodiazepine use and overall prescription drug use, we excluded benzodiazepines when enumerating prescription drugs. We did not attempt to ascertain history of previous medication use or duration of use of current drugs. Alcohol and smoking data were collected starting in Wave 2, ascertaining both current and lifetime use.
Prescription drugs were classified and sub-classified according to therapeutic category using a scheme based on the American Hospital Formulary System (AHFS) .15 Among drugs classified as sedative-hypnotic and anti-anxiety agents, those belonging to the benzodiazepine class were identified for the current analysis. For these analyses, we assumed sustained use when individuals reported using benzodiazepines at two consecutive biennial waves.
Participants were assessed using the modified Center for Epidemiologic Studies-Depression score (mCES-D) in which scores can range from 0-20 representing the number of depressive symptoms experienced over most of the preceding week.16 As no separate anxiety scale was administered, two mCES-D items (“I felt fearful” and “I was bothered by things that don’t usually bother me”) were selected on the basis of face validity to serve as proxy measurements for anxiety. To avoid collinearity between the overall mCES-D and the two anxiety items within the same scale, we removed these two items and entered them as separate covariates. Scores on the remaining items were summed and treated as a continuous variable representing number of depression symptoms.
Potential explanatory variables examined included age, sex, education, total number of prescription medications (categorized at the median as <2 or >=2), self-rated health (fair/poor vs. good /excellent), ever smoked, current alcohol use, sleeping difficulties, the two “anxiety” items from the mCES-D each examined separately, and the total of the remaining mCES-D items.
Descriptive statistics used to characterize benzodiazepine users at Wave 2 included Chi-square, Fisher’s exact test, or Mann-Whitney U test comparing the above characteristics between users and non-users.
The outcome variable in the analytic model was “sustained” benzodiazepine use at two consecutive waves. We combined data from multiple waves to investigate the associations between the explanatory variables at each wave and sustained benzodiazepine use at the subsequent two waves, using longitudinal lag-time models with generalized estimating equations (GEE).17, 18 This method models the outcome variable at multiple time points subsequent to the time the explanatory variable was measured, and adjusts for within-subject correlations across measurement points. Thus, we examined variables at Wave 2 as predictors of sustained benzodiazepine use at Waves 3 and 4, variables at Wave 3 to predict sustained benzodiazepine use at Waves 4 and 5, etc. The explanatory variables were treated as time-dependent covariates.
RESULTS
The original cohort consisted of 1,681 individuals. Current analyses begin with Wave 2 data because several of the key variables were measured for the first time at this wave. At Waves 2, 3, 4, 5, and 6 there were 1,342, 1,166, 1,017, 846, and 665 participants respectively. Mortality was the main source of attrition, as would be expected in an aging cohort (9%-15% between 2-year waves); dropouts, relocation, and other sources of attrition were an average of 2.8% between waves (i.e., 1.4% per year).
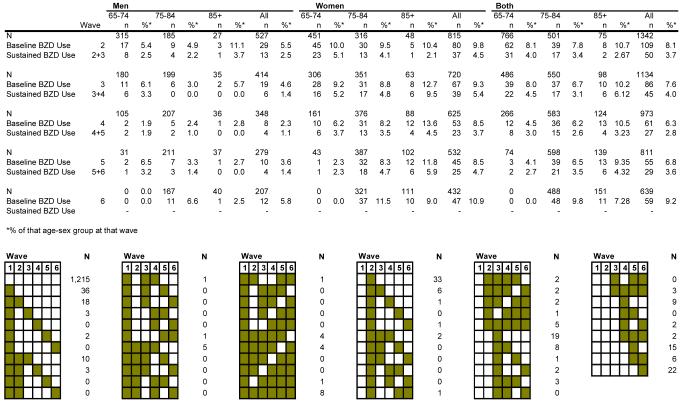
Figure 1 includes both a frequency distribution and a graphic. The frequency of use and sustained use is tabulated for each wave, overall and subdivided by age and gender. For example, at Wave 2, there were 109 overall users of benzodiazepines and 50 sustained users (i.e., individuals who reported using benzodiazepines at both Waves 2 and 3). The graphic illustrates the various frequency patterns of individuals’ benzodiazepine use over the entire study. Each column represents a wave (from left to right, Wave 1 through Wave 6); each row represents a drug use pattern with the shaded cells indicating waves in which a benzodiazepine was taken. The top row depicts subjects who did not take benzodiazepines at any wave, totaling 1,215. The second row shows individuals who took benzodiazepines only at Wave 1, but not at any subsequent wave (n=36). The third row represents individuals who reported benzodiazepine use at two consecutive waves, Wave 1 and Wave 2 (n=18) but not at Waves 3 to 6, and so on. Initially, 5.5 % of men and 9.8 % of women were using benzodiazepines. Approximately 50%, 44%, and 25% of these users aged 65-74. 75-84, and 85+ were sustained users at follow-up.
Figure 1. Frequency of Baseline and Sustained Benzodiazepine Use at Each Wave.
Each column represents a data collection wave (from left to right, Wave 1 through Wave 6); each row represents a drug use pattern with the shaded cells indicating waves in which a benzodiazepine was taken. For example: Row 1 indicates that 1215 participants reported no benzodiazepine use throughout the study. Row 2 shows 36 individuals were taking benzodiazepines at Wave 1 but not subsequently. Row 3 shows 18 participants were taking benzodiazepines at both Waves 1 and 2, but not subsequently. Row 4 shows 3 individuals were taking benzodiazepines at Wave 1 and Wave 3 only.
Across waves, the two most commonly used benzodiazepines were alprazolam (22% - 37%) and lorazepam (18% - 36%). Longer-acting benzodiazepines, such as clonazepam and diazepam, were used less frequently (3% - 12%). Also of note, the proportion of individuals using the sedative-hypnotic temazepam increased over time from 5.9% in 1987-1989 to 36.7% in 1998-2000.
Chi-square tests (Table 1) showed that benzodiazepine users at Wave 2 were significantly more likely than non-users to be female, less highly educated, have more depressive symptoms, report both anxiety symptoms, use more non-benzodiazepine prescription medications, have lower self-rated health, and have more difficulty staying asleep. Users of benzodiazepines at Wave 2 were also significantly less likely to consume alcohol. Notably, no individuals taking benzodiazepines were also taking antidepressants.
Table 1. Wave 2 Characteristics Among Benzodiazepine Users and Nonusers*.
| Benzodiazepine Use | |||||
|---|---|---|---|---|---|
| Wave 2 characteristics | Total (N=1,342) | Users (N=109) | Nonusers (N=1233) | P value⊥ | |
| Age | 0.70 | ||||
| 65-74 | 766 (57.1%) | 62 (56.9%) | 704 (51.7%) | ||
| 75-84 | 501 (37.3%) | 39 (35.8%) | 462 (37.5%) | ||
| 85+ | 75 (5.6%) | 8 (7.3%) | 67 (5.4%) | ||
| Gender | 0.005 | ||||
| Males | 527 (39.3%) | 29 (26.6%) | 498 (40.4%) | ||
| Females | 815 (60.7%) | 80 (73.4%) | 735 (59.6%) | ||
| Education | 0.04 | ||||
| <High school | 540 (40.2%) | 54 (49.5%) | 486 (39.4%) | ||
| ≥High school | 802 (59.8%) | 55 (50.5%) | 747 (60.6%) | ||
| Number of Depressive Symptoms‡ | 1.32 ± 2.45 | 2.88 ± 3.94 | 1.18 ± 2.22 | <0.001 | |
| Feel fearful | <0.001 | ||||
| No | 1263 (95.9%) | 95 (88.8%) | 1168 (96.5%) | ||
| Yes | 54 (4.1%) | 12 (11.2%) | 42 (3.5%) | ||
| Bothered | 0.02 | ||||
| No | 1228 (93.2%) | 94 (87.9%) | 1134 (93.7%) | ||
| Yes | 89 (6.8%) | 13 (12.1%) | 76 (6.3%) | ||
| Total Number of Prescription Drugs Excluding Benzodiazepines | <0.001 | ||||
| None | 386 (28.8%) | 12 (11.0%) | 374 (30.3%) | ||
| 1 | 292 (21.8%) | 0 (0.0%) | 292 (23.7%) | ||
| ≥2 | 664 (49.5%) | 97 (89.0%) | 567 (46.0%) | ||
| Self-Rated Health | <0.001 | ||||
| Fair/Poor | 300 (22.6%) | 45 (42.1%) | 255 (20.9%) | ||
| Excellent/Good | 1026 (77.4%) | 62 (57.9%) | 964 (79.1%) | ||
| Ever Smoked | 0.10 | ||||
| No | 1197 (89.9%) | 93 (85.3%) | 1104 (90.3%) | ||
| Yes | 135 (10.1%) | 16 (14.7%) | 119 (9.7%) | ||
| Current Alcohol Consumption | 0.001 | ||||
| <Once a month | 833 (73.3%) | 82 (87.2%) | 751 (72.0 %) | ||
| ≥Once a month | 304 (26.7%) | 12 (12.8%) | 292 (28.0%) | ||
| Difficulty Falling Asleep | 0.06 | ||||
| Never/Rarely | 829 (62.5%) | 55 (51.9%) | 774 (63.4%) | ||
| Sometimes | 371 (28.0%) | 39 (36.8%) | 332 (27.2%) | ||
| Usually/Always | 127 (9.6%) | 12 (11.3%) | 115 (9.4%) | ||
| Sleep Continuity Disturbance | 0.003 | ||||
| Never/Rarely | 942 (71.0%) | 60 (56.6%) | 882 (72.3%) | ||
| Sometimes | 303 (22.9%) | 37 (34.9%) | 266 (21.8%) | ||
| Usually/Always | 81 (6.1%) | 9 (8.5%) | 72 (5.9%) | ||
| Early Morning Awakening | 0.23 | ||||
| Never/Rarely | 1073 (80.9%) | 81 (76.4%) | 992 (81.3%) | ||
| Sometimes | 203 (15.3%) | 18 (17.0%) | 185 (15.2%) | ||
| Usually/Always | 50 (3.8%) | 7 (6.6%) | 43 (3.5%) | ||
| Excessive Daytime Somnolence | 0.73 | ||||
| Never/Rarely | 1110 (83.8%) | 86 (81.1%) | 1024 (84.0%) | ||
| Sometimes | 206 (15.6%) | 19 (17.9%) | 187 (15.3%) | ||
| Usually/Always | 9 (7.7%) | 1 (1.0%) | 8 (0.7%) | ||
| Wave 2 Benzodiazepine Use | |||||
| No | 1233 (91.9%) | ||||
| Yes | 109 (8.1%) | ||||
For some variables, the categories do not add up to 1 due to rounding.
P value is based on the univariable analysis using chi-square or Fisher’s exact test for categorical variables and Mann-Whitney U test for continuous variables.
mCES-D total score minus the two anxiety items: “I felt fearful” and “I was bothered by things that do not usually bother me,” which are included as separate covariates.
As would be expected, benzodiazepine use at any wave was highly correlated with sustained benzodiazepine use at subsequent waves (OR: 32.23; p<0.001). Given this high degree of collinearity, current benzodiazepine use was not included in the multivariable model predicting future use.
The longitudinal lag-time models revealed the factors at a given baseline wave X that predicted sustained benzodiazepine use at the two subsequent waves X+1 and X+2 (Table 2). In the univariable (unadjusted) analyses, only three variables at baseline were significantly associated with future sustained benzodiazepine use: female gender (p=0.001), use of two or more prescription medications (p=0.002), and ever smoked (p=0.001). In the multivariable model, adjusting for covariates using backwards stepwise selection, the same three variables remained significant after adjustment and were independently associated with subsequent sustained use: female gender (p=0.049), use of two or more prescription medications (p=0.001), and ever smoked (p=0.01).
Table 2. Univariable and Multivariable Longitudinal Lag-time Analysis on Sustained Benzodiazepine Use.
| Univariable | Multivariable* | ||||||
|---|---|---|---|---|---|---|---|
| Baseline characteristics | Odds Ratio |
95% Confidence Interval |
P value | Odds Ratio |
95% Confidence Interval |
P value | |
| Age | 65-74 | 1.00 | |||||
| 75-84 | 0.66 | (0.42, 1.05) | 0.08 | ||||
| 85+ | 0.37 | (0.09, 1.51) | 0.17 | ||||
| Gender | Males | 1.00 | 1.00 | ||||
| Females | 4.13 | (1.80, 9.46) | 0.001 | 2.57 | (1.002, 6.57) | 0.049 | |
| Education | <High school | 1.00 | |||||
| ≥High school | 1.87 | (1.00, 3.52) | 0.051 | ||||
| Number of Depressive Symptoms | 1.08 | (0.97, 1.21) | 0.18 | ||||
| Feel Fearful | No | 1.00 | |||||
| Yes | 0.61 | (0.05, 7.68) | 0.70 | ||||
| Bothered | No | 1.00 | |||||
| Yes | 1.25 | (0.41, 3.82) | 0.69 | ||||
| Total Number of Prescription Drugs | None | 1.00 | 1.00 | ||||
| Excluding Benzodiazepines | 1 | 1.11 | (0.34, 3.59) | 0.86 | 1.18 | (0.36, 3.84) | 0.79 |
| ≥2 | 4.93 | (1.88, 12.92) | 0.001 | 5.20 | (1.95, 13.86) | 0.001 | |
| Self-Rated Health | Fair/Poor | 1.00 | |||||
| Excellent/Good | 1.003 | (0.57, 1.76) | 0.99 | ||||
| Ever Smoked | No | 1.00 | 1.00 | ||||
| Yes | 2.82 | (1.49, 5.33) | 0.001 | 3.01 | (1.32, 6.85) | 0.01 | |
| Current Alcohol Consumption | <Once a month | 1.00 | |||||
| ≥Once a month | 0.72 | (0.45, 1.15) | 0.17 | ||||
| Difficulty Falling Asleep | Never/Rarely | 1.00 | |||||
| Sometimes/ Usually/Always | 1.25 | (0.79, 1.97) | 0.34 | ||||
| Sleep Continuity Disturbance | Never/Rarely | 1.00 | |||||
| Sometimes/ Usually/Always | 0.91 | (0.62, 1.31) | 0.59 | ||||
| Early Morning Awakening | Never/Rarely | 1.00 | |||||
| Sometimes/Usually/Always | 0.93 | (0.53, 1.61) | 0.79 | ||||
| Excessive Daytime Somnolence | Never/Rarely | 1.00 | |||||
| Sometimes/Usually/Always | 0.89 | (0.62, 1.27) | 0.52 | ||||
| Baseline Benzodiazepine Use * | No | 1.00 | |||||
| Yes | 32.23 | (17.67, 58.78) | <0.001 | ||||
Baseline benzodiazepine use was not included in the multivariable model selection procedure because of multicollinearity.
DISCUSSION
In this population-based epidemiologic study, benzodiazepine users at Wave 2 were significantly more likely to be women, to consume alcohol less frequently, to be less educated, to use more prescription drugs, and to report more depressive symptoms, anxiety symptoms, poorer self-rated health, and difficulty staying asleep. In longitudinal lag-time analysis, sustained benzodiazepine use over two consecutive waves (four years) was independently associated with female gender and two other variables at the preceding wave two years earlier: use of two or more non-benzodiazepine prescription medications, and smoking.
Our finding that women were significantly more likely than men to be sustained benzodiazepine users has particular clinical relevance because of the increased falls risk with benzodiazepine use and the elevated risk of fractures in elderly women.19 Sustained users were also likely to be taking two or more other prescription medication, reflecting greater medical (including psychiatric) burden and greater risk for anxiety disorders.20, 21 Those taking a greater number of medications are also at elevated risk of drug-drug interactions, e.g., additive side effects of medications, such as sedation and confusion, and also for delirium and agitation which can be mistaken for anxiety and lead to prescription of benzodiazepines.22
We did not find an association between age and benzodiazepine use, perhaps because of the age truncation of our cohort (65 and older) as was also the case in an elderly community dwelling population in France.9 Prior studies which found benzodiazepine use to be associated with age were conducted in individuals across the life span.23, 24 There also was no association in our sample between self-reported depression symptoms and sustained benzodiazepine use, although users were more likely than non-users to report presence of the two “anxiety” questions in the unadjusted analyses. A previous study found that use of sedative-hypnotic and anxiolytic medications was correlated with female gender, white race, depressive symptoms, and poor self-rated health. The odds for using these medications and being white in that biracial cohort were 4.7.25 Our cohort was almost entirely white.
Not surprisingly since benzodiazepines are commonly prescribed for insomnia, we found sleep complaints reported more frequently by benzodiazepine users than non-users. However, the significant difference was in sleep continuity disturbance (difficulty staying asleep, or intermittent insomnia), rather than with difficulty falling asleep (initial insomnia). Potentially, benzodiazepines can provide symptom relief for initial insomnia but cause REM rebound leading to intermittent insomnia.26
Among our participants, a history of cigarette smoking was significantly associated with sustained use of benzodiazepines. In the French community study, smoking was associated with baseline benzodiazepine use, but not with sustained use.9 It is possible that smokers are using cigarettes to self-medicate the same anxiety for which they are taking benzodiazepines. Alternatively, the activating effects of nicotine could increase arousal and anxiety levels, prompting benzodiazepine use. A Greek clinic-based study found an association between benzodiazepine use and cigarette smoking, but it is unclear which began first.27 We found no other studies suggesting that benzodiazepines were being used to counter the activating effects of cigarette smoking.
A novel finding was that sustained users of benzodiazepines were less likely to report alcohol consumption, in the unadjusted analyses. Potentially, alcohol and benzodiazepines produce comparable effects on baseline anxiety and sleep, leading individuals to feel the need for one or the other but not both.
Awareness of patients’ characteristics predicting long-term use patterns can inform providers’ prescribing behavior. However, providers’ own characteristics, not examined in our study, might also influence their prescription practices. Canadian studies have shown that physicians who prescribe long-acting benzodiazepines for the elderly are themselves likely to be older, to be generalists, and to practice in long-term-care settings.28 Further, older specialist practitioners were more likely to prescribe long-acting benzodiazepines to male patients.29 Such information suggests possible physician demographics to target for continuing medical education around benzodiazepine prescription.
Advantages of our study include the use of a large cohort of community-dwelling elderly individuals in the United States who were followed at 2-year intervals for a period of up to 12 years. Since recruitment was based on age-stratified random sampling from the electoral rolls of a stable population, the cohort is representative of the population from which it was drawn; thus, results are generalizable to other populations of largely white older adults of relatively low socioeconomic status. Our lag-time analytic models allowed us to identify factors associated with future sustained use of benzodiazepines, adjusting for one another and also for within-subject correlations across waves.
As these data were not primarily collected for the purposes of studying drug use as the outcome, we had no data on drug abuse or dependence which are of growing importance as the US population ages. Medication data were obtained via self-report and confirmatory observation of the patient’s prescription bottles, but we were not able to assess adherence. Further, we did not use an established anxiety measure but rather used two items from the depression scale as proxies for anxiety. We assumed sustained use when individuals reported using benzodiazepines at consecutive waves, but could not confirm whether their use had in fact been continuous.
CONCLUSION
While there are considerable benefits to using benzodiazepines in treating a number of conditions, practitioners also must maintain an awareness of the potential adverse effects, especially in the elderly. Additionally, some insurance programs now impose significant restrictions on the use of benzodiazepines or refuse to cover such medications. This is particularly true of Medicare Part D, which excludes any coverage for benzodiazepines.30 Therefore, it is important for the prescriber to be aware of factors that may lead to sustained use. Knowledge of characteristics that are associated with such use can be helpful to the clinician when making decisions about starting or continuing an older adult on benzodiazepines.
ACKNOWLEDGMENT
The authors would like to thank Dr. Ihsan M. Salloum and Dr. Laurie A. Lavery for helpful comments on the manuscript, all MoVIES personnel over the duration of the study, and all MoVIES participants.
The work reported here was performed in part with support from research grant R01 AG07562 from NIA and career development grants K24 AG022035 from NIA and DK 59928 from NIDDK.
Footnotes
CONFLICT OF INTERST
The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
This work was presented as a poster at the Early Investigators’ Session at the American Association for Geriatric Psychiatry Annual Meeting in March of 2008.
REFERENCES
- 1.Sadock BJ, Sadock VA. Kaplan & Sadock’s Synopsis of Psychiatry. Lippincott Williams & Wilkins; Philadelphia: 2003. [Google Scholar]
- 2.Llorente MD, David D, Golden AG, Silverman MD. Defining patterns of benzodiazepine use in older adults. J Geriatr Psychiatry Neurol. 2002;13:150–160. doi: 10.1177/089198870001300309. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association . Benzodiazepine dependence, toxicity, and abuse. American Psychiatric Association; Washington, DC: 1990. [Google Scholar]
- 4.Tamblyn R, Abrahamowicz M, du Berger R, et al. A 5-year prospective assessment of the risk associated with individual benzodiazepines and doses in new elderly users. J Am Geriatr Soc. 2005;53:233–241. doi: 10.1111/j.1532-5415.2005.53108.x. [DOI] [PubMed] [Google Scholar]
- 5.Grad RM. Benzodiazepines for insomnia in community-dwelling elderly: A review of benefit and risk. J Fam Pract. 1995;41:478–481. [PubMed] [Google Scholar]
- 6.Paterniti S, Dufouil C, Alperovitch A. Long-term benzodiazepine use and cognitive decline in the elderly: The Epidemiology of Vascular Aging Study. J Clin Psychopharmacol. 2002;22:285–293. doi: 10.1097/00004714-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Voyer P, McCubbin M, Cohen D, et al. Unconventional indicators of drug dependence among elderly long-terms users of benzodiazepines. Issues Ment Health Nurs. 2004;25:603–628. doi: 10.1080/01612840490472138. [DOI] [PubMed] [Google Scholar]
- 8.Wagner AK, Zhang F, Soumerai SB, et al. Benzodiazepine use and hip fractures in the elderly: who is at greatest risk? Arch Intern Med. 2004;164:1567–1572. doi: 10.1001/archinte.164.14.1567. [DOI] [PubMed] [Google Scholar]
- 9.Fourrier A, Letenneur L, Dartigues JF, et al. Benzodiazepine use in an elderly community-dwelling population. Eur J Clin Pharmacol. 2001;57:419–425. doi: 10.1007/s002280100326. [DOI] [PubMed] [Google Scholar]
- 10.Lechevallier-Michel N, Beer C, Fourrier-Reglat A. Incidence and characteristics of benzodiazepine use in an elderly cohort: The EVA study. Therapie. 2005;60:561–566. doi: 10.2515/therapie:2005078. [DOI] [PubMed] [Google Scholar]
- 11.Soumerai SB, Simoni-Wastila L, Singer C, et al. Lack of relationship between long-term use of benzodiazepines and escalation to high dosages. Psychiatr Serv. 2003;54:1006–1011. doi: 10.1176/appi.ps.54.7.1006. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett G, Abrahamowicz M, Tamblyn R, et al. Longitudinal patters of new benzodiazepine use in the elderly. Pharmacoepidemiol Drug Saf. 2004;13:669–682. doi: 10.1002/pds.908. [DOI] [PubMed] [Google Scholar]
- 13.Ganguli M, Belle S, Ratcliff G, et al. Sensitivity and specificity for dementia of population-based criteria for cognitive impairment: The MoVIES project. J Gerontol A Biol Sci Med Sci. 1993;48:M152–M161. doi: 10.1093/geronj/48.4.m152. [DOI] [PubMed] [Google Scholar]
- 14.Mulsant B, Ganguli M, Seaberg E. The relationship between self-rated health and depressive symptoms in an epidemiological sample of community-dwelling older adults. J Am Geriatr Soc. 1997;45:954–958. doi: 10.1111/j.1532-5415.1997.tb02966.x. [DOI] [PubMed] [Google Scholar]
- 15.Lassila HC, Stoehr GP, Ganguli M, et al. Use of prescription medications in an elderly rural population: The MoVIES project. Ann Pharmacother. 1996;30:589–595. doi: 10.1177/106002809603000604. [DOI] [PubMed] [Google Scholar]
- 16.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 17.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 18.Zeger SL, Liang K-Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 19.Cumming RG, Le Couteur DG. Benzodiazepines and risk of hip fractures in older people: A review of the evidence. CNS Drugs. 2003;17:825–837. doi: 10.2165/00023210-200317110-00004. [DOI] [PubMed] [Google Scholar]
- 20.Jonas BS, Franks P, Ingram DD. Are symptoms of anxiety and depression risk factors for hypertension? Arch Fam Med. 1997;30:11–22. doi: 10.1001/archfami.6.1.43. [DOI] [PubMed] [Google Scholar]
- 21.Harris E, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. E1998. [DOI] [PubMed] [Google Scholar]
- 22.Mancuso C, Tanzi M, Gabay M. Paradoxical reactions to benzodiazepines: Literature review and treatment options. Pharmacotherapy. 2004;24:1177–1185. doi: 10.1592/phco.24.13.1177.38089. [DOI] [PubMed] [Google Scholar]
- 23.Neutel CI. The epidemiology of long-term benzodiazepine use. Int Rev Psychiatry. 2005;17:189–197. doi: 10.1080/09540260500071863. [DOI] [PubMed] [Google Scholar]
- 24.Lagnaoui R, Depont F, Fourrier A, et al. Patterns and correlates of benzodiazepine use in the French general population. Eur J Clin Pharmacol. 2004;60:523–529. doi: 10.1007/s00228-004-0808-2. [DOI] [PubMed] [Google Scholar]
- 25.Blazer D, Hybels C, Simonsick E, et al. Sedative, hypnotic, and antianxiety medication use in an aging cohort over ten years: A racial comparison. J Am Geriatr Soc. 2000;48:1073–1079. doi: 10.1111/j.1532-5415.2000.tb04782.x. [DOI] [PubMed] [Google Scholar]
- 26.Chouinard G. Issues in the clinical use of benzodiazepines: Potency, withdrawal, and rebound. J Clin Psychiatry. 2004;65(suppl 5):7–12. [PubMed] [Google Scholar]
- 27.Lekka NP, Paschalis C, Beratis S. Nicotine, caffeine and alcohol use in high- and low-dose benzodiazepine users. Drug Alcohol Depend. 1997;45:207–212. doi: 10.1016/s0376-8716(97)01362-8. [DOI] [PubMed] [Google Scholar]
- 28.Monette J, Tamblyn RM, McLeod PJ, et al. Characteristics of physicians who frequently prescribed long-acting benzodiazepines for the elderly. Eval Health Prof. 1997;20:115–130. doi: 10.1177/016327879702000201. [DOI] [PubMed] [Google Scholar]
- 29.Egan M, Wolfson C, Moride Y, et al. Do patient factors alter the relationship between physician characteristics and use of long-acting benzodiazepines? J Clin Epidemiol. 2000;53:1181–1187. doi: 10.1016/s0895-4356(00)00243-2. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Medicare & Medicaid Services . Summary of HR 1: Medicare Prescription Drug, Improvement, and Modernization Act of 2003. Centers for Medicare & Medicaid Services; Washington, DC: 2004. Public Law 108-173. [Google Scholar]