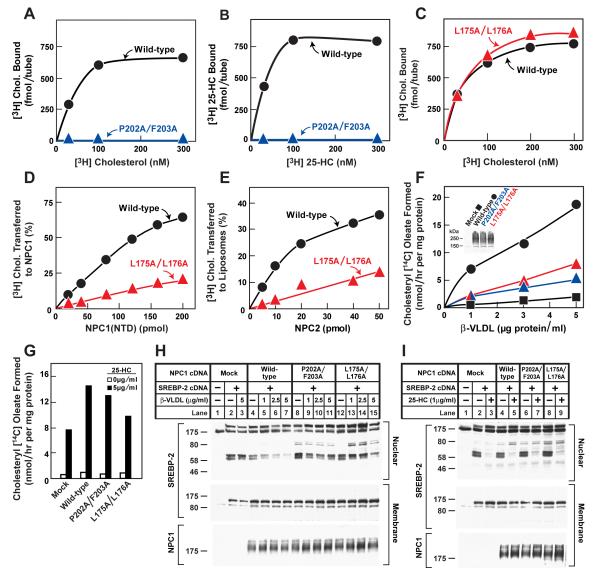
Figure 5. Biochemical and Functional Analysis of Sterol Binding and Transfer Mutants.
(A-C) 3H-Sterol binding. Each reaction, in a final volume of 80 μl buffer C with 0.004% NP-40, contained 220 ng purified WT or mutant NPC1(NTD)-LVPRGS-His8-FLAG, 1 μg BSA, and indicated concentration of [3H]cholesterol (132 dpm/fmol) (A,C) or [3H]25-HC (165 dpm/fmol) (B). After incubation for 24 hr at 4°C, the amount of bound 3H-sterol was measured as described in Supplemental Experimental Procedures. Each value is the average of duplicate assays and represents total binding after subtraction of a blank value (10-70 fmol/tube). Mean variation for each of the duplicate assays in A, B, and C were 6.1%, 5.0%, and 5.0%, respectively.
(D) [3H]Cholesterol transfer from NPC2 to NPC1(NTD). Each reaction, in a final volume of 200 μl buffer D (pH 5.5) without detergent, contained ∼40 pmol of donor protein NPC2-FLAG complexed to [3H]cholesterol (830 fmol, 132 dpm/fmol) and increasing concentrations of purified WT or mutant NPC1(NTD)-LVPRGS-His8-FLAG acceptor protein. After incubation for 15 min at 4°C, the amount of [3H]cholesterol transferred to NPC1(NTD) was measured by Ni-NTA-agarose chromatography as described in the [3H]cholesterol transfer assay in Experimental Procedures. Each value is the average of duplicate assays and represents percentage of [3H]cholesterol transferred to NPC1(NTD). The 100% value for transfer from NPC2 was 830 fmol/tube. Mean variation for each of the duplicate assays for WT and mutant were 7.9% and 8.2%, respectively
(E) [3H]Cholesterol transfer from NPC1(NTD) to liposomes as a function of NPC2. Each reaction, in final a volume of 200 μl buffer D (pH 5.5) without detergent, contained ∼50 pmol of WT or L175A/L176A versions of NPC1(NTD)-LVPRGS-His8-FLAG, each complexed to [3H]cholesterol (950 and 660 fmol, respectively; 132 dpm/fmol); 20 μg PC liposomes labeled with Texas red dye; and increasing concentrations of NPC2-His10. After incubation for 10 min at 4°C, the amount of [3H]cholesterol transferred to liposomes was measured in the flow-through of the nickel column as described for the [3H]cholesterol transfer assay in Experimental Procedures. Each value is the average of duplicate assays and represents the percentage of [3H]cholesterol transferred to liposomes. Blank values in the absence of NPC2 (5-6% transfer) were subtracted. The 100% values for transfer from WT and L175A/176A versions of NPC1(NTD) were 950 and 660 fmol/tube, respectively. Mean variation for each of the duplicate assays for WT and mutant were 8.8% and 9.3%, respectively.
(F-I) Cholesterol esterification and SREBP-2 processing in mutant CHO cells lacking NPC1 function transfected with NPC1 cDNAs. Mutant CHO 4-4-19 cells were set up for experiments and transfected with 2 μg pcDNA3.1 or with WT or mutant versions of pCMV-NPC1-His8-FLAG (F,G); or co-transfected with 0.4 μg pcDNA3.1 or with WT or mutant versions of pTK-NPC1-His8-FLAG3 plus 3 μg pTK-HSV-BP2 (H,I) as described in Experimental Procedures. 24 hr after transfection, the medium was switched to medium A containing 5% newborn calf lipoprotein-deficient serum, 5 μM compactin, and 50 μM sodium mevalonate. After incubation for 24 hr, the medium was switched to the same medium containing 50 μM compactin and various concentrations of ß-VLDL (F,H) or 25-HC (G,I) as indicated.
(F,G) Cholesterol esterification. After incubation for 5 hr at 37°C, each cell monolayer was pulse-labeled for 1 hr with 0.2 mM sodium [14C]oleate (6301 dpm/pmol). The cells were then harvested for measurement of their content of cholesteryl [14C] oleate and [14C] triglycerides. Each value is the average of duplicate incubations. Mean variation for each of the duplicate incubations for WT, P202A/F203A, and L175A/L176A were 9.6%, 14.5%, and 4.3%, respectively. The rate of synthesis of [14C]triglycerides for mock, NPC1 WT, NPC1(P202A/F203A), and NPC1(L175A/L176A) transfected cells incubated with 5 μg/ml ß-VLDL was 340, 396, 352, and 365 nmol/hr per mg protein, respectively. The rate of synthesis of [14C]triglycerides incubated with 25-HC was 347, 496, 304, and 434 nmol/hr per mg protein, respectively.
(H,I) SREBP-2 processing. After incubation for 4 hr at 37°C, cells received a direct addition of 25 μg/ml of N-acetyl-leucinal-leucinal norleucinal. After 1 hr, triplicate dishes were harvested and pooled for preparation of nuclear extracts and 100,000 g membrane fractions, which were analyzed by immunoblotting for the indicated protein. The concentrations of antibodies were 0.2 and 4 μg/ml for SREBP-2 (anti-HSV) and NPC1 (anti-FLAG), respectively. All filters were exposed on x-ray film for 2-10 s.
(A-I) Similar results were obtained in 3 or more independent experiments.