Fig. 2.
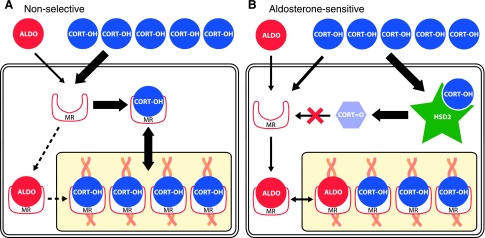
In most mineralocorticoid receptor (MR)-expressing cells (A), the primary ligand is corticosterone or cortisol (both designated “CORT”). These glucocorticoids circulate in the blood plasma at 1,000-fold higher concentrations than aldosterone (ALDO). This systemic imbalance between CORT and ALDO is further exaggerated in the brain, at least in rodents, where only a minute fraction of circulating aldosterone penetrates the BBB (49, 136, 138). Thus, in most MR-expressing cells, basal occupation of this receptor by glucocorticoids prevents the binding of aldosterone, which despite an equivalent binding affinity, may be 10-fold more potent in producing transcriptional activation once it is bound to the MR (9). In contrast, cells that express 11β-hydroxysteroid dehydrogenase type 2 (HSD2; B) can convert a large fraction of intracellular CORT to an 11-oxo metabolite with greatly reduced MR-binding affinity. Aldosterone is not a substrate for HSD2, so this mechanism confers cellular sensitivity to physiological concentrations of this hormone. HSD2 is only a partial filter, leaving a significant amount of unaltered corticosterone able to bind the MR (49, 54), probably because of its greater affinity for the MR (Km <1 nM) than HSD2 (Km ∼10 nM in rat).