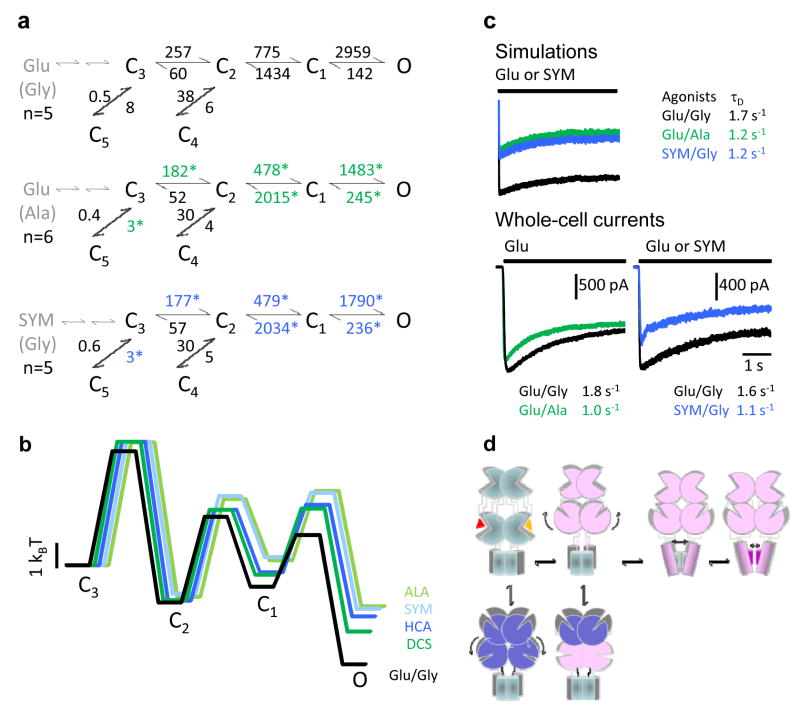
Figure 5. Kinetic mechanism of NMDA receptor activation by partial agonists.
a, State models with rate constants represented as averages of results from fits to individual records in each condition. Open states are represented as an aggregate O, for simplicity. Two sequential binding steps are represented in grey. Asterisks denote rates which were significantly different from Glu/Gly (p < 0.05). b, Calculated free-energy relationships were plotted with respect to C3. States C4 and C5, which represent desensitized conformations, were omitted from the energy diagram to emphasize the activation pathway. Traces were slightly shifted horizontally for clarity. c, Top, current traces simulated with the models and rate constants shown in panel a, and after appending two agonist-binding steps to state C3 (see Methods). Bottom, whole-cell voltage-clamp current traces in response to 5-s agonist-applications as indicated, in the continuous presence of the corresponding co-agonist. Overlaid traces represent averages of 5 interleaved sweeps recorded from the same cell. Time constants of fits with single exponential functions are given for the displayed traces. d, Cartoon representing rearrangements in quaternary structure possibly corresponding to the kinetic intermediates characterized in this study. The activation pathway is imagined to consist of: the aggregated closures of all ligand-binding domains (C3-C2), followed by movements in trans-membrane helices or/and the linker regions (C2-C1), and finally direct gating of the permeation pathway (C1-O) by fast oscillations of residues in the selectivity filter. Off-pathway intermediates result from non-productive closures of the ligand-binding domains, fostered perhaps by concurrent rearrangements in the N-terminal domains. This model takes into account our previous observation that glutamate dissociates with measurable rates from state C3 but not others (ref 24), and the current results that partial agonists affect to the same extent and in a symmetrical manner the C3-C5 and C3-C2 equilibria. It also incorporates current knowledge on the role of: the N-terminal domains in desensitization, the third transmembrane domain in activation and the selectivity filter in gating.