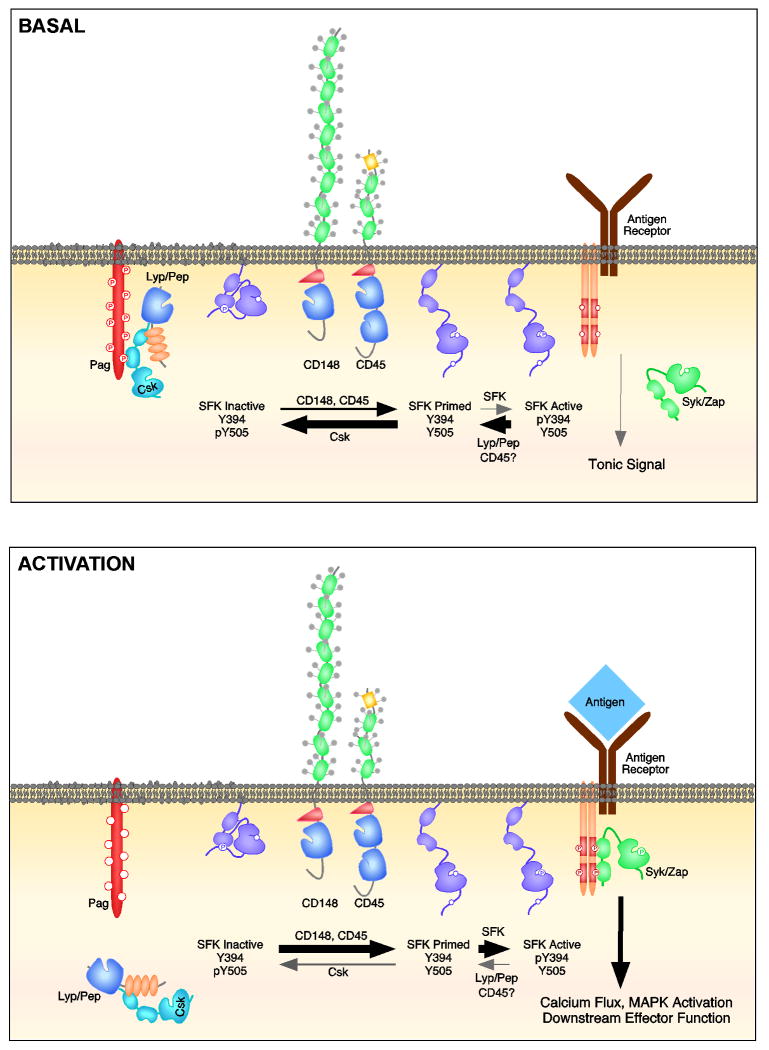
Figure 3. Reciprocal regulation of SFK activation by CD45, CD148, and Lyp/Pep.
SFKs are in a dynamic equilibrium between their inactive and active conformations in both the basal state and during immune cell activation (while B cell activation is depicted, similar mechanisms likely exist in other hematopoietic cell lineages). In the basal state, phosphorylation of the adapter PAG facilitates recruitment of Csk and Lyp/Pep. Csk phosphorylates the negative regulatory tyrosine while Lyp/Pep dephosphorylates the autocatalytic tyrosine. This results in the SFK adopting a closed conformation. By opposing Csk and dephosphorylating the negative regulatory tyrosine, CD45 and CD148 generate a pool of SFKs in an open or ‘primed’ conformation that can be rapidly activated should antigen be encountered. We hypothesize that low levels of SFK transphosphorylation of the autocatalytic site occur in the basal state, which is necessary for tonic signaling and cell survival. This is balanced by the action of PAG/Csk/Pep (and potentially CD45 targeting either directly or indirectly the autocatalytic tyrosine). During antigen encounter and receptor activation, PAG becomes dephosphorylated via mechanisms that are incompletely elucidated (but may involve CD45), Csk and Lyp/Pep dissociate from the cell surface leaving CD45 and CD148 actions on the negative regulatory tyrosine unopposed. Phosphorylation of the autocatalytic tyrosine is now favored. The fully active SFK can phosphorylate the ITAMs of the immunoreceptor, facilitating recruitment of Syk/Zap70 and amplification of downstream signaling cascades.