Abstract
Background:
Myocardial fibers are grouped into lamina (or sheets) 3-4 cells thick. Fiber shortening produces systolic LV wall thickening primarily by laminar extension, thickening and shear, but the regional variability and transmural distribution of these three mechanisms are incompletely understood.
Methods and Results:
Nine sheep had transmural radiopaque markers inserted into the anterior basal and lateral equatorial LV. 4D marker dynamics were studied with biplane videofluoroscopy to measure circumferential, longitudinal, and radial systolic strains in the epicardium, midwall, and endocardium. Fiber and sheet angles from quantitative histology allowed transformation of these strains into transmural contributions of sheet extension, thickening, and shear to systolic wall thickening. At all depths, systolic wall thickening in the anterior basal region was 1.6-1.9 times that in the lateral equatorial region. Interestingly, however, systolic fiber shortening was identical at each transmural depth in these regions. Endocardial anterior basal sheet thickening were >2x greater than in the lateral equatorial region (epicardium: 0.16±0.15 vs. 0.03±0.06; endocardium: 0.45±0.40 vs. 0.17±0.09). Midwall sheet extension was >2x that in the lateral wall (0.22±0.12 vs. 0.09±0.06). Epicardial and midwall sheet shears in the anterior wall were ∼2x higher than in the lateral wall (epicardium: 0.14±0.07 vs. 0.05±0.03; midwall: 0.21±0.12 vs. 0.12±0.06).
Conclusions:
These data demonstrate fundamentally different regional contributions of laminar mechanisms for amplifying fiber shortening to systolic wall thickening. Systolic fiber shortening was identical at each transmural depth in both the anterior and lateral LV sites. However, systolic wall thickening of the anterior site was much greater than that of the lateral site. Fiber shortening drives systolic wall thickening, but sheet dynamics and orientations are of great importance to systolic wall thickening. LV wall thickening and its clinical implications pivot on different wall thickening mechanisms in various LV regions. Attempts to implant healthy contractile cells into diseased hearts or surgically manipulate LV geometry will need to take into account not only cardiomyocyte contraction, but also transmural LV intercellular architecture and geometry.
Keywords: wall thickening, strains, fibers and sheets, myocardial contraction
INTRODUCTION
Left ventricular wall thickening is a significant contributor to stroke volume. Although myocardial fiber contraction provides the cellular basis for regional myocardial wall thickening, 15% fiber shortening only leads to an 8% increase in myocyte diameter, which cannot explain the observed >40% radial LV wall thickening and >60% ejection fraction (1,2). Myocardial fibers have been shown to be grouped into laminar “sheets”, 3-4 cells thick, which are interconnected by an extensive extracellular matrix (3,4). Spotnitz et al. first suggested that reorientation of transmural sheets of fibers could provide a basis for wall thickness changes (5). LeGrice et al. (3,4) showed that longitudinal-radial shear of these sheets was likely to be an important mechanism underlying systolic wall thickening. Costa et al. (1) extended this work, showing that systolic sheet extension and sheet-normal shear were the primary determinants of systolic wall thickening. Despite these important findings, the regional distribution and mechanisms of systolic wall thickening within the left ventricular myocardium remains poorly understood. The objective of this study was to examine the regional variability and transmural distribution of fiber-sheet strains and their contribution to systolic wall thickening.
METHODS
All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (DHEW NIHG publication 85-23, revised 1985). This study was approved by the Stanford Medical Center Laboratory Research Animal Review Committee and conducted according to Stanford University policy. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written. The surgical preparation and marker data acquisition methods have been described in detail previously (6-10).
Surgical Preparation
Nine adult Dorsett-hybrid sheep were premedicated with ketamine (25mg/kg i.m.) intubated and ventilated. Anesthesia was maintained with inhalation isoflurane (1-2.5%) The heart was exposed through a left thoracotomy and thirteen subepicardial helical tantalum radiopaque markers were surgically implanted to silhouette the left ventricular chamber (Figure 1A). Epicardial echocardiography was used to identify a segment of the mid-lateral equatorial LV wall between the papillary muscles and in the anterior LV wall basal to the anterior papillary muscle. After the measurement of wall depth of these two regions, three transmural columns of beads (three 0.7mm diameter gold beads implanted from endocardium to epicardium and one 1.7mm bead sewn onto the epicardial surface above each column, Figure 1B) were then implanted into these two segments using a bead insertion trocar oriented normal to the regional epicardial tangent plane.
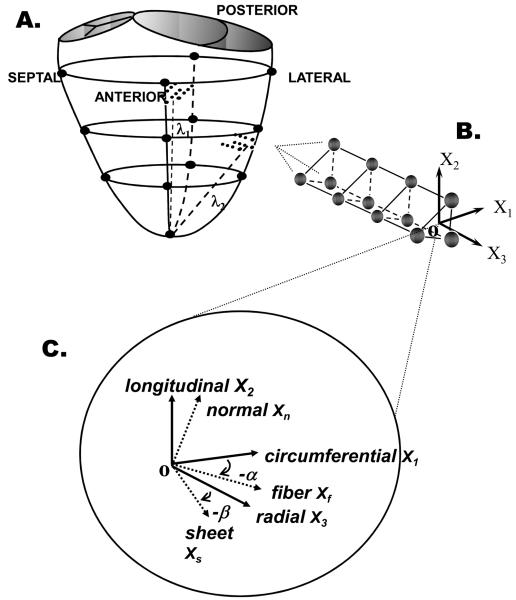
FIGURE 1.
A. Locations of LV epicardial markers (large filled circles), LV lateral equatorial and anterior basal transmural beadsets (small filled circles). B. Transmural tissue blocks for histological measurements were excised from each heart at the anterior basal and lateral equatorial regions immediately below the transmural beadsets, with the edges cut parallel to X1, X2, and X3 , the circumferential, longitudinal and radial cardiac axes, respectively. C. Transmural fiber angles (α) were measured from sections cut parallel to X1-X2 plane. At a given wall depth, measured fiber angles (α) and sheet angles (β) were used to define local fiber-sheet coordinates with basis vectors of fiber (Xf) axis, sheet axis perpendicular to Xf within sheet plane (Xs), and axis normal to the sheet plane (Xn). These same measurements were obtained from the anterior wall tissue block.
Data acquisition
Immediately post-operation the animals were taken to the catheterization laboratory. Each animal was placed in the right lateral decubitus position, kept ventilated and anesthesia was maintained with inhalation isoflurane (1-2.5%). A micromanometer-tipped catheter (Model MPC-500, Millar Instruments, Houston, TX) calibrated in a 37°C water bath was inserted into the LV via a carotid artery catheter. Philips Optimus 2000 biplane Lateral ARC 2/Poly DIAGNOST C2 system (Philips Medical Systems, North America Company, Pleasanton, CA, USA) was used to acquire biplane videofluoroscopic (60Hz) images of all radiopaque markers and beadsets. Two dimensional images from two radiographic views were digitized and merged to yield 3-D coordinates for each marker and bead for every frame (i.e. every 16.7ms). Simultaneous biplane videofluoroscopy, left ventricular pressure, aortic pressure and ECG signal were recorded in steady state, baseline open-chest condition with the heart in sinus rhythm and ventilation briefly arrested at end-expiration.
Three consecutive steady-state beats in sinus rhythm were selected for analysis from each study. For each cardiac cycle, end-diastole (ED) was defined as the videofluoroscopic frame immediately prior to the upstroke of the LV pressure curve, defined by dLVP/dt > 120 mmHg/s. End-systole (ES) was defined as the videofluoroscopic frame when d2LVP/dt2 changed sign from minus to plus, a definition which captures the onset of relaxation.
Following the study, conventional 3.0 mm perfusion balloon catheters (GUIDANT AguilTrac Peripheral Catheter, Santa Clara, CA) were advanced in the proximal left anterior descending and circumflex arteries. An intravenous bolus of sodium pentothal (1g i.v.) was given and the heart was arrested at end-diastole with an intravenous potassium chloride bolus (80mEq). LV pressure was adjusted by blood withdrawal to match the previous in vivo LV end diastolic pressure, defined as the pressure immediately preceding the upstroke of LV pressure curve in the beating heart. To fix the heart in situ, 300ml of buffered glutaraldehyde (5%) was infused simultaneously into the both anterior descending and left circumflex coronary arteries. The heart was then explanted and stored in 10% formalin for later fiber and sheet angle and wall thickness examination.
Histological measurements
A transmural rectangular block of myocardial tissue, directly contiguous and basal to the implanted marker columns (Figure 1B), was removed from the ventricular wall from both anterior basal and lateral equatorial regions, with the edges of the block cut parallel to the local circumferential (X1), longitudinal (X2), and radial (X3), (Figure 1B) axes of the left ventricle. Each block was sliced into sequential 1-mm-thick sections parallel to the X1-X2 plane, thereby providing a series of slices from epicardium to endocardium for measurement of fiber angle (α). The fiber angle (α), defined as the angle between the local muscle fiber axis (Xf) and circumferential axis (X1 Figure 1C), was measured at five sites on each image using image-processing software (SPOT Advanced Version 4.0.1, Diagnostic Instruments, Inc., Sterling Heights, MI). Mean α was used to characterize the fiber angle at each transmural depth. Two parallel cuts separated by approximately 1-mm were then made normal to the fiber axis in each of these transmural sections. The samples were kept moist with a 30% sucrose solution to avoid the distortional effects of dehydration and to minimize freezing artifact during direct histological measurements of sheet angle beta (β) from the sheet normal plane. The fiber-normal slices were placed in 15mm × 15 mm × 5 mm plastic molds (Tissue-Tek, Cryomold Intermediate, Miles Inc., Elkhart, IN), embedded in OCT compound (Tissue-Tek, Sakura Finetek USA Inc., Torrance, CA), frozen over dry ice, then stored for 2-4 days in a −80° freezer. They were then cut into 8-10μm thick sections using a cryostat (Jung Frigocut 2800 N, Leica Inc, Germany) and transferred to a glass slide where they were imaged immediately with a digital camera (RT Color, 1X HRD 100-NIK, Diagnostic Instruments, Inc., Sterling Heights, MI) mounted on a light microscope (Leica Type 301-371.010, Leica Inc., Germany) at 25x magnification. Myolaminae coursing in the direction noted from the frozen specimen were observed (sheets) and, over a one-minute period, gaps between the cleavage planes appeared between the myolaminae. Using image-processing software, five β angles were measured between sheet orientations (Xs) and X3 normal to the endocardial face, over the length of the specimen. Mean β was used to characterize the sheet angle at each transmural depth.
Strain Analysis
Transmural Cardiac Strains
Transmural bead placement enabled the assessment of myocardial deformations of LV wall in the lateral equatorial and anterior basal regions. For each beat, the reference (undeformed) state was taken at ED for that beat, and the ensuing positions of the beads for that beat were defined by displacement from their positions at ED as characterized by a continuous polynomial position field with quadratic dependence in X3 and bilinear dependence in X1 and X2 using least-squares minimization. For each time-sample analyzed: X3 (radial axis) was defined normal to the epicardial tangent plane created by the three epicardial surface beads, with origin (O) at the centroid of these three epicardial beads (Figure 1); X2 (longitudinal axis) was defined at the intersection of the epicardial plane with a plane containing X3 and a line (long axis λ1 (anterior wall) and λ2 (lateral wall) from the apex marker through the origin [long axis λ1 aligns within 8° of the long axis defined by Streeter (9)]; and X1 (circumferential axis) was defined normal to X2 and X3. In cardiac coordinates (X1, X2, X3), the three normal strain components measure local myocardial stretch or shortening along the circumferential (E11), longitudinal (E22), and radial (E33) cardiac axes. The three shear strains (E12, E13, and E23) represent angle changes between pairs of the originally orthogonal coordinate axes. Strains were interpolated along the centroid of the bead columns at 1% increments of wall depth from the epicardium to the most subendocardial bead. Cardiac normal and shear strains were calculated at 20%, 50%, and 80% depths from the epicardium, with ED as the reference configuration and ES as the deformed configuration for both anterior and lateral LV wall. More detailed strain analysis has been previously provided (6,8). Table 1 provides the symbols and abbreviations used.
TABLE 1.
SYMBOLS AND ABBREVIATIONS USED
| α | Fiber angle (degrees) |
| β | Sheet angle (degrees) |
| E33 | Radial systolic (LV wall thickening) strain |
| Eff | Fiber strain |
| Ess | Sheet extension strain |
| Enn | Sheet normal (thickening) strain |
| Efs | Fiber-sheet shear |
| Efn | Fiber-sheet normal shear |
| Esn | Sheet-sheet normal shear |
| Exxc | Component of radial systolic (LV wall thickening) strain |
Transmural Fiber-Sheet Strains
In each heart, at each transmural depth, cardiac finite strains (i.e., relative to X1, X2, and X3, Figure 1) were transformed into sheet strains at that depth oriented along the fiber, sheet and sheet-normal axes by application of the fiber (α) and sheet (β) angle measurements in that heart at that depth as:
| (1) |
Subsequently fiber sheet strains calculated included shortening or stretch along the fiber (Eff), sheet (Ess) and sheet-normal (Enn) directions and three shear strains (Efn, Efs, Esn). Next, the contributions of sheet strains to radial thickening (E33 strain) were calculated as follows:
| (1,7) |
Statistical Analysis
Data was initially investigated to check for normality. Non-normal data was transformed with a log-log transformation prior to further statistical analysis. All data were compared using repeated-measures ANOVA (Sigmastat 3.11, SPSS, Inc. Chicago, IL). P<0.05 was considered statistically significant. Data are reported as mean ±1SD unless otherwise stated.
RESULTS
Table 2 shows group mean anterior basal and lateral equatorial LV transmural fiber angles (α) and sheet angles (β) measured at 20%, 50% and 80% wall depth from the epicardium. Fiber angles (α) varied linearly with depth and are identical at both regions. Sheet angles (β) exhibited pleated sheet behavior with alternating signs between 20%, 50% and 80% depths with a roughly mirror image in the two LV regions. These findings are consistent with those in other hearts from our previous study (9).
TABLE 2.
TRANSMURAL FIBER AND SHEET ANGLES
| Fiber Angle (α°) | Sheet Angle (β°) | |||||
|---|---|---|---|---|---|---|
| subepicardium | midwall | subendocardium | subepicardium | midwall | subendocardium | |
|
Anterobasal wall |
−37±6 | −8±7 | 24±11 | −55±10 | 43±5 | −52±10 |
|
Lateral equatorial wall |
−37±8 | −8±9 | 21±10 | 46±7 | −37±4 | 53±8 |
Group mean (±SD) data from 9 hearts. Fiber angle α=angle between X1 and Xf; Sheet angle β=angle between X3 and XS (See Text and Figure 1 for details).
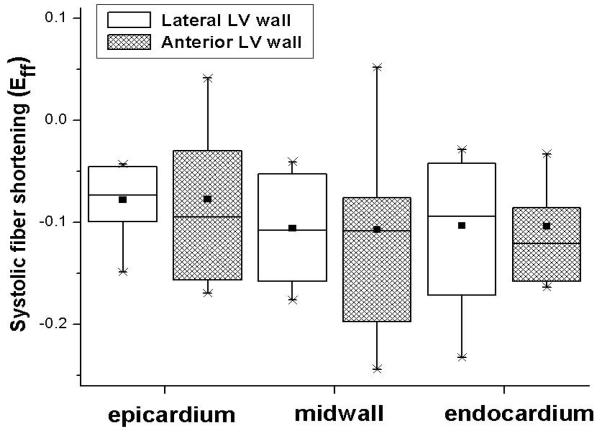
Systolic fiber-sheet strains in both LV regions are summarized in Table 3. Eff was uniformly negative (fiber shortening) and identical in both regions at each transmural depth (Table 3 and Figure 2). Subepicardial and midwall sheet-normal shear (Esn) and subendocardial fiber-sheet shear (Efs) differed significantly between the regions in the anterior and lateral LV wall. Anterior wall subendocardium sheet thickening (Enn) was also significantly greater than that of the lateral LV wall. Midwall sheet extension (Ess) in the anterior wall was double that of the lateral wall.
TABLE 3.
TRANSMURAL SYSTOLIC FIBER-SHEET STRAINS IN THE ANTEROBASAL AND LATERAL EQUATORIAL REGION AT THREE DIFFERENT LV WALL DEPTHS
| N=9 | Subepicardium | Midwall | Subendocardium | |||
|---|---|---|---|---|---|---|
| Anterior | Lateral | Anterior | Lateral | Anterior | Lateral | |
| Eff | −0.08±0.07 | −0.08±0.04 | −0.11±0.09 | −0.11±0.05 | −0.10±0.05 | −0.10±0.08 |
| Ess | 0.00±0.04 | 0.11±0.07 | 0.22±0.12 | 0.09±0.06* | −0.06±0.12 | −0.04±0.20 |
| Enn | 0.16±0.15 | 0.03±0.06 | 0.02±0.12 | 0.08±0.10 | 0.45±0.40 | 0.17±0.09* |
| Efs | −0.03±0.05 | −0.01±0.07 | −0.03±0.04 | 0.02±0.03 | 0.00±0.05 | 0.10±0.05* |
| Efn | 0.03±0.09 | 0.01±0.02 | −0.03±0.04 | −0.07±0.05 | −0.06±0.10 | 0.04±0.05 |
| Esn | −0.14±0.07 | 0.05±0.03* | 0.21±0.12 | −0.12±0.06* | −0.19±0.23 | 0.20±0.15 |
Group mean (±SD) data from 9 hearts. ED = reference configuration; ES = deformed configuration.. Eff = Fiber strain; Ess = Sheet strain; Enn = Strain normal to laminae; Efs = Fiber-Sheet shear; Efn = Fiber-Normal shear; Esn = Sheet-Normal shear.
p<0.05 lateral vs. anterior fiber-sheet absolute strain values at the same wall depth; All data between the anterior and lateral LV wall at different levels were compared using repeated-measures ANOVA.
FIGURE 2.
Boxplot of systolic fiber shortening (Eff) in lateral equatorial (open bars) and anterior basal (hatched bars) LV regions. Data from 9 hearts. Abscissa: Wall depth. No statistically significant differences were noted. All data between the anterior and lateral LV wall at different levels were compared using repeated-measures ANOVA. Each boxplot displays the smallest value (lowest point on vertical whisker), the 25th percentile (bottom of box), the median (horizontal line in box), the group mean (square black symbol in the box), 75th percentile (top of box), and largest value (highest point on vertical whisker).
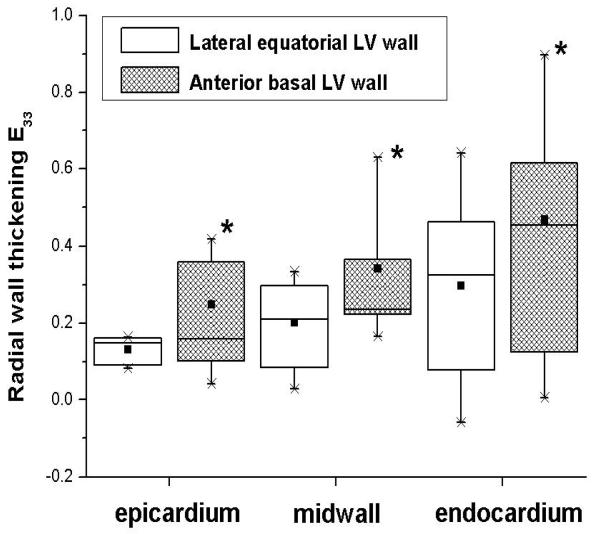
Table 4 summarizes the transmural components of systolic wall thickening. At each depth radial wall thickening of the anterior basal wall was significantly greater than that of the lateral equatorial wall (Figure 3). Epicardial and midwall sheet shear (Esn) wall thickening contribution in the anterior wall was two times greater than that in the lateral wall (Table 4, Figure 4). Moreover, the epicardial and endocardial anterior basal sheet thickening (Enn) contributions to wall thickening were greater than two times that in the lateral wall.
TABLE 4.
TRANSMURAL COMPONENTS OF SYSTOLIC WALL THICKENING IN LATERAL EQUATORIAL AND ANTEROBASAL REGION.
| N=9 | Anterior wall | Lateral wall | |
|---|---|---|---|
| subepicardium | E2033 | 0.25±0.15 | 0.13±0.03* |
| E20ssc | 0.00±0.02 | 0.06±0.04 | |
| E20nnc | 0.12±0.11 | 0.02±0.04* | |
| E20snc | 0.13±0.06 | 0.05±0.03* | |
| midwall | E5033 | 0.34±0.17 | 0.20±0.12* |
| E50ssc | 0.12±0.08 | 0.06±0.05 | |
| E50nnc | 0.02±0.07 | 0.03±0.04 | |
| E50snc | 0.20±0.12 | 0.11±0.06* | |
| subendocardium | E8033 | 0.47±0.30 | 0.30±0.24* |
| E80ssc | −0.02±0.06 | 0.00±0.08 | |
| E80nnc | 0.31±0.30 | 0.12±0.09* | |
| E80snc | 0.19±0.23 | 0.18±0.15 | |
Group mean (±SD) data from 9 hearts.
p<0.05 from repeated-measures ANOVA of lateral LV wall data compared with anterior LV wall data
E2033, E5033, E8033 = Systolic radial strain at 20, 50, 80% wall depth from epicardium.
E20ssc, E50ssc, E80ssc = SS component of systolic radial strain at 20, 50, 80% wall depth.
E20nnc, E50nnc, E80nnc = NN component of systolic radial strain at 20, 50, 80% wall depth.
E20snc, E50snc, E80snc = SN component of systolic radial strain at 20, 50, 80% wall depth.
FIGURE 3.
Boxplot of radial systolic wall thickening (E33) in the lateral equatorial (open boxes) and anterior basal (hatched boxes) LV wall. Data from 9 hearts. Abscissa: Wall depth. *p<0.05 from repeated-measures ANOVA between anterior and lateral E33 at each depth. Each boxplot displays the smallest value (lowest point on vertical whisker), the 25th percentile (bottom of box), the median (horizontal line in box), the group mean (square black symbol in the box), 75th percentile (top of box), and largest value (highest point on vertical whisker).
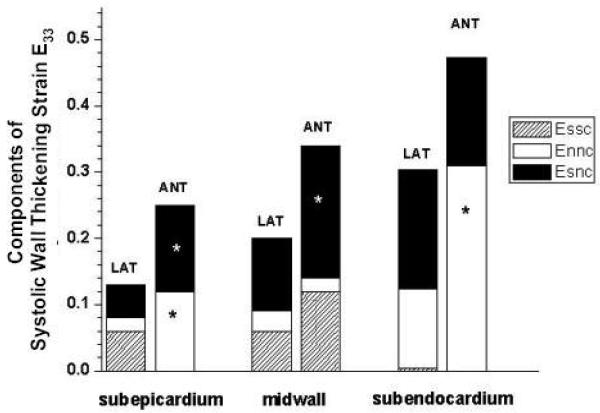
FIGURE 4.
Components of systolic wall thickening in lateral basal and anterior equatorial LV wall. Group mean data (n=9) from Table 4. Ordinate: Systolic strain (ED reference state; ES deformed state); Abscissa: Wall depth. Each pair of bars displays data at lateral (LAT) and anterior (ANT) wall. Total height of each bar is the transmural systolic wall thickening strain (E33). Sheet components of E33 (derived from the equation: E33 = EssCos2 β + EnnSin2 β + 2EsnSinβCosβ are bar heights for sheet extension (Essc, hatched), sheet thickening (Ennc open), and sheet-normal shear (Esnc filled). *p<0.05 from repeated-measures ANOVA between anterior and lateral wall at the same wall depth.
Figure 4 illustrates the group mean data summing the systolic wall thickening sheet components in the lateral and anterior LV regions. In the lateral LV wall, sheet shear (Esn) was always an important component of systolic wall thickening at all transmural depths (38% in the epicardium, 55% in the midwall and 60% in the endocardium). Sheet thickening (Enn) was a major wall thickening component in the endocardium (40%) and sheet extension (Ess) in the epicardium (46%).
In the anterior LV wall, sheet shear (Esn) remained a significant component of systolic wall thickening at all transmural depths (52% in the epicardium, 58% in the midwall and 40% in the endocardium). Sheet thickening (Enn) was a major wall thickening component in the epicardium (48%) and endocardium (66%), with sheet extension (Ess) more important in the midwall (35%).
DISCUSSION
The principle findings of this study were: 1) Group mean radial wall thickening in the anterobasal region was 1.6 to 1.9 times that of the lateral equatorial region; 2) Group mean systolic fiber shortening, however, was identical at each transmural depth in each region; 3) Fundamental heterogeneity exists in regional contributions of laminar mechanisms for amplifying fiber shortening to systolic wall thickening; and, 4) Fiber orientations are similar between these regions, but sheet orientations differ widely. Thus, fiber shortening drives systolic wall thickening, but sheet dynamics, orientations and, likely, intercellular connections are of great importance to systolic wall thickening.
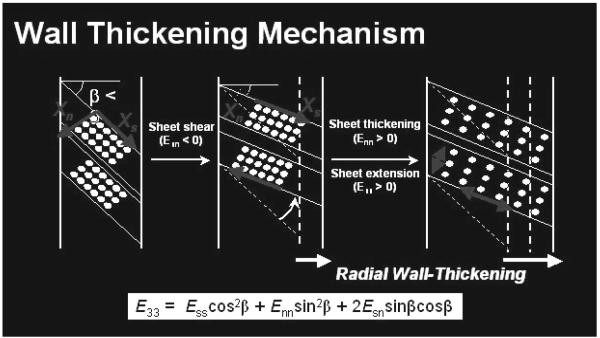
Systolic LV radial wall thickening (see review in Ref 8) is an important component of normal LV function because of its substantial contribution to stroke volume and its sensitivity to hypoperfusion and altered metabolism (11-13). Present clinical approaches to evaluate regional ventricular function are based mainly on wall thickening. Although myocardial fiber contraction provides the basic mechanics for regional myocardial wall thickening, 15% fiber shortening along the long axis only leads to an 8% increase in myocyte diameter. Yet 40% radial LV wall thickening and 60% ejection fraction are typical observed (1,2). Myocardial fibers have been shown to be organized into laminar “sheets”, approximate 3-4 cells thick, interconnected by an extensive extracellular matrix that provides various degrees of myocyte coupling within each sheet and between adjacent sheets. It was first suggested that reorientation of transmural sheets of fibers could provide a basis for LV wall mechanics during relaxation and contraction (5). Subsequently, it was demonstrated that reorientation of longitudinal-radial cleavage planes due to transverse shear could account for the majority of end-systolic wall thickening strain (3). Costa et al. (1) then showed that systolic sheet extension and sheet-normal shear could also be primary determinants of systolic wall thickening (Figure 5).
FIGURE 5. Components of Systolic LV wall thickening.
A schematic model relating sheet geometry to systolic wall thickening. Fibers are grouped into sheets, 3-4 cells thick. Sheet shear (Esn) measures the sliding of adjacent sheets relative to one another, with the resulting sheet reorientation in the radial direction producing wall thickening. The radial components of sheet extension (Ess) and thickening (Enn) also produce wall thickening.
Placement of the transmural beadsets allows measurement of transmural cardiac strains along the longitudinal, circumferential and radial axis. By performing direct microstructural measurements (7,9) in the region of the beadsets, these cardiac strains can be transformed into fiber and sheet strains and these transforms can be utilized to characterize wall thickening components through the LV wall.
Transmural radial wall thickening in the anterobasal wall was found to be considerably greater than that of the lateral equatorial wall. However systolic fiber shortening was identical at each transmural depth in both regions. To more fully comprehend this phenomenon, we examined the fiber and sheet strain contributions to systolic wall thickening.
In these ovine hearts, systolic fiber shortening (Eff) was 8–11% (Table 3) and did not exhibit a transmural gradient, which is consistent with previous predictions and observations (1,14). Interestingly, as seen in previous canine (1) and human (15) studies, sheet sheet-normal shear Esn in these ovine hearts greatly exceeded fiber-sheet normal shear Efn (Table 3). This preference for sheet sliding relative to one another along the sheet direction, rather than between fibers within the sheets, may reflect a tighter coupling of myocytes by extensive collagen networks within the sheets and a looser coupling between adjacent sheets.
In the lateral LV wall, similar to our previous findings (7), the present study showed that sheet shear (Esn) is always an important component of systolic wall thickening at all transmural depths. Furthermore, sheet thickening (Enn) is dominant in the endocardium, while sheet extension (Ess) contributes importantly in the epicardium. In the anterior basal LV wall, similar to the findings of Costa et al. (1) in the same region, sheet shear remains a dominant component of systolic wall thickening at all transmural depths. Enn is positive (sheet thickening) and contributes significantly to wall thickening in both the epicardium and endocardium. This finding however differs from that reported by Costa et al. (1), where Enn was small and negative (sheet thinning) at the anterior basal site. We believe sheet thickening is probably more likely during systole, while sheet thinning is more difficult to explain and may require cellular interdigitation. It is possible that the thinning they observed could result, in part from the small βs measured by their indirect histological approach in canine hearts (1); while we measured much larger β values with our direct histological approach applied to the same region in ovine hearts. Moreover, a different species (canine vs. ovine) was used in their study, which may also contribute to the differences in sheet orientation and strains between the two studies.
Using diffusion tensor MRI to obtain sheet structure and strain rate, Dou et al. (15) reported mid-systolic sheet dynamics at the equatorial LV level in normal human hearts and also found substantial contributions of Ess, Esn, and Enn to E33 in the lateral and anterior wall. Similar to our findings, they demonstrated that a major contribution to radial thickening was associated with sheet-related strains, and that the three components of fiber-related strains contributed less to radial thickening. Their study, however differed from the present study, by reporting average contributions over the entire transmural thickness at each LV site due to resolution limitations. The present study suggests that considerable transmural sheet geometry and strains remain to be revealed by MRI as its spatial resolution increases.
With these data describing the heterogeneity in sheet contribution to systolic wall thickening, the next question was, “what accounts for these differences in the two regions?” In order to answer this question, the structural differences, i.e. fiber and sheet orientations were explored.
The present study, applying a direct measurement approach to the ovine anterior basal and lateral equatorial LV wall, demonstrated that fiber angles varied linearly with depth (Table 2), as seen in previous studies (7,9). This helical myofiber orientation is relatively preserved among different species (1,16-19), ranging from approximately −60° to +60° from epicardium to endocardium, i.e. from a left-handed helix in the epicardium to a right-handed helix in the endocardium, with circumferential fibers at midwall. More importantly, in the present study, the transmural distribution of fiber angles was found to be similar in both the anterior and lateral LV regions. Along with identical systolic fiber shortening, fiber orientation and fiber shortening cannot explain the regional differences in systolic wall thickening.
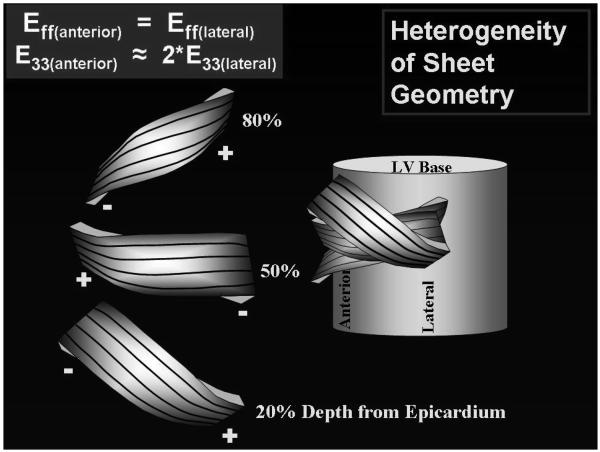
A dramatic difference in the sheet angles in these two regions were found, as illustrated in Figure 6 and Table 2, with one being the mirror image of the other across the LV wall in the epicardium, midwall and endocardium. This is consistent with our previous study (7,9) where sheet angles fell into two distinct families: β− and β+. In the lateral equatorial wall, near the epicardium, β belonged to the β+ family; near the midwall, to the β− family; and near the endocardium, again to the β+ family. In the anterior basal wall, the reverse trend was observed; near the epicardium, β belonged to the β− family; near the midwall, to the β+ family; and near the endocardium, again to the β− family. Figure 6 presents a conceptual schematic model depicting one way the results observed in these three regions can be synthesized into a more global picture of the cardiac microstructure.
FIGURE 6. Fiber and Sheet Orientations.
A model synthesizing fiber and sheet data from anterior and lateral equatorial regions of the heart. The illustration on the left represent sheets at 20, 50, and 80% wall depth from the epicardium. Black lines on the surface of the sheets represent measured fiber angles (α) qualitatively, with negative α values near the epicardium, 0° at midwall, and positive α values near the epicardium. Left margins of the sheets represent data from the anterior wall, with sheets belonging to the - family at 20%, to the + family at 50%, and to the - family at 80% wall depth. Right region of the sheets represent data from the lateral region, with sheets belonging to the + family at 20%, to the - family at 50%, and to the + family at 80% wall depth from the epicardium.
Described previously (9), this accordion-like laminar distribution allows alternating shear displacements to occur within the wall that reduces the shear displacements required of the epicardium relative to the endocardium. With this mechanism, shear deformations can be roughly the same throughout the wall, but the direction of sheet sliding alternates.
While distribution of α for these hearts (Table 2) was virtually indistinguishable from that observed in the canine basal anterior wall (1), distributions of β were distinctly different. Costa et al. (1), with indirect measurements, reported mean basal anterior wall β values of 0° at 20% depth to −20° at 50% and 80% depths from the epicardium. We found, by direct measurement, much greater magnitudes of β in a pleated-accordion pattern, ranging from a mean of −55° at 20% depth to +43β at 50% depth and then back to −52° at 80% depth (Table 2).
Chen et al. (20), using diffusion tensor MRI with isolated rat hearts, found that sheet angles changed from 36° at end diastole to 20° at end systole, reflecting a change in sheet orientation during ventricular contraction. Their observations provide further evidence that fiber and sheet geometry alterations during systole provide a fundamental mechanism for regional systolic LV wall thickening.
Rohmer et al. (21), using DT-MRI, found that fiber angles vary smoothly across the LV wall from epicardium (negative angles) to endocardium (positive angles) in human hearts, similar to our findings and previous canine and ovine studies (7, 9). They also attempted to identify the sheet model, which is broadly similar to that proposed by Legrice et al. (4) and Costa et al. (1) where the sheet architecture is complex, with sheet orientations depending on myocardial location.
In summary, these data demonstrate fundamentally different regional contributions of laminar mechanisms for amplifying fiber shortening to systolic wall thickening. Systolic fiber shortening was identical at each transmural depth in both the anterior and lateral LV sites, yet systolic wall thickening of the anterior site was 60-90% greater than that of the lateral site. Fiber shortening drives systolic wall thickening, but sheet dynamics and sheet geometry are of great importance to systolic wall thickening. In other words, myocyte contraction contributes to radial wall thickening and ventricular ejection both by myocyte shortening and by the related secondary induction of changes in fiber and sheet organization. This mechanism will depend critically on the orientation of the myocytes and their interaction with the extracellular matrix throughout the ventricular wall during systole.
The complexity of this mechanism of wall thickening suggests that abnormalities in either the contractile unit (fiber and sheet) or the infrastructure (extracellular matrix) can dramatically affect wall thickening. The characterization of the baseline three-dimensional myocardial architecture and dynamics are important, as collagen degradation can be brought about by disease states (22), and altered baseline myocyte infrastructure may be a key mechanism in ventricular dysfunction(17,23).
Normal cardiac microstructure and systolic strains are tightly coupled, and deviations could result in apoptosis and matrix remodeling (24-29). Recent studies indicate that developed ventricular wall stress is very sensitive to changes in fiber and sheet structure over the cardiac cycle (30-32). Thus it is conceivable that evaluation of preclinical abnormalities of ventricular function could be improved by incorporating quantitative data on fiber and sheet structure in systole. Furthermore, characterization of such structural changes in diseased hearts may facilitate the investigation of the mechanisms of structural and functional adaptations in the ventricular remodeling process.
The enhanced understanding of the myocardial fibrous and laminar architecture, transmural LV strains and LV wall mechanics could contribute significantly to the design of better surgical remodeling procedures to restore normal ventricular strain patterns in patients with cardiomyopathy. Sheet geometry, strains and intercellular matrix coupling are crucial for LV wall thickening and dynamics. The present study suggests that attempts to implant healthy contractile cells into diseased hearts, or surgically manipulate cardiac geometry, must take into account, not only the contraction of cardiac cells, but of equal importance, their orientation and transmural coupling, which may be specific to each ventricular region.
LIMITATIONS
Considerable caution is warranted before extrapolation of these results to the human heart. Fiber angle measurements (α) are relatively straightforward and reproducible, but sheet angle measurements (β) are more difficult. As discussed above, it is quite possible that there are major species as well as regional differences in transmural sheet angle measurements (although this may only strengthen the concept that variations in the macrostructure of the wall are important in determining wall thickening). Multiple β populations are found at different wall depths, particularly in the subendocardium. It is not appropriate to average these populations, because they are often of opposite sign and could average, inappropriately, to zero. Our approach in the present study, as in other previous studies(1,7,9,33), was to measure and employ the dominant β population in our analysis, assuming that the predominant orientation would have the major effect on LV wall thickening. But future work is needed to understand the functional importance of regions with multiple β populations.
While myocardial deformations are directly measured in this study, fiber and sheet strains are calculated from a structural model of the LV wall. The spatial resolution of the present study is insufficient to allow inferences regarding individual cell or sheet shape changes during systole, although measurement of such changes will be an important, but very difficult future goal. It should also be emphasized that collagen content and geometry or elasticity of the intracellular matrix were not measured in this study.
Our observations are limited only to the lateral equatorial and anterior basal ovine LV wall. However, it should be emphasized that the findings of this study show, despite identical regional systolic fiber shortening, regional sheet dynamics and orientations different importantly within the left ventricle and are very important to systolic wall thickening and LV function. The results of the present study encourage a thorough and systematic study of the entire LV. Placement of transmural beadsets is invasive. MRI will ultimately be the best method for this type of study. It is non-invasive and it will allow assessment of the entire LV, rather than a few specific regions. However, the current spatial resolution of MRI is not sufficient to make the required measurements directly. Most MRI studies have reported average strain contributions over the entire transmural thickness at each LV site or used model assumptions for each LV wall depth.
ACKNOWLEDGEMENTS
We appreciate the superb technical assistance provided by Mary K. Zasio, B.A., Maggie Brophy, A.S. and Mark Grisedale, D.V.M.
FUNDING SOURCES
Supported by Grants HL-29589 and HL-67025 from the National Heart, Lung and Blood Institute. Doctors Cheng and Nguyen were Carl and Leah McConnell Cardiovascular Surgical Research Fellows. Doctor Cheng and Nguyen were recipients of the Thoracic Society Foundation Research Fellowship Award.
Footnotes
Presented in part at the American Heart Association Scientific Sessions 2005. Causes and Contributors to LV Dysfunction. Dallas, TX.
Clinical Perspective
Left ventricular wall thickening is a significant contributor to stroke volume. Although myocardial fiber contraction provides the cellular basis for regional myocardial wall thickening, 15% fiber shortening only leads to an 8% increase in myocyte diameter, which cannot explain the observed >40% radial LV wall thickening and >60% ejection fraction. Myocardial fibers have been shown to be grouped into laminar “sheets”, 3-4 cells thick, which are interconnected by an extensive extracellular matrix. This study demonstrates fundamentally different regional contributions of laminar mechanisms for amplifying fiber shortening to systolic wall thickening. Systolic fiber shortening was identical at each transmural depth in both the anterior and lateral LV sites, but systolic wall thickening of the anterior site was much greater than that of the lateral site. This implies that sheet geometry and dynamics and the exact nature of their coupling by the extracellular matrix are of great importance to systolic wall thickening. The complexity of this mechanism of wall thickening suggests that abnormalities in either the contractile unit (fiber and sheet) or the infrastructure (extracellular matrix) can dramatically affect wall thickening. The characterization of the baseline three-dimensional myocardial architecture and dynamics are important, as collagen degradation can be brought about by disease states, and altered baseline myocyte infrastructure may be a key mechanism in ventricular dysfunction. Enhanced understanding of myocardial fibrous and laminar architecture coupling to transmural LV strains and LV wall mechanics could contribute significantly to the design of better surgical remodeling procedures to restore normal ventricular strain patterns in patients with cardiomyopathy. Further, attempts to implant healthy contractile cells or tissue-engineered constructs into diseased hearts, or surgically manipulate cardiac geometry, must take into account, not only the contraction of cardiac cells, but, of equal importance, their orientation and transmural coupling, which may be specific to each ventricular region and transmural depth.
CONFLICT OF INTERESTS DISCLOSURES
None.
Reference List
- 1.Costa KD, Takayama Y, McCulloch AD, Covell JW. Laminar fiber architecture and three-dimensional systolic mechanics in canine ventricular myocardium. Am J Physiol. 1999;276:H595–H607. doi: 10.1152/ajpheart.1999.276.2.H595. [DOI] [PubMed] [Google Scholar]
- 2.Sonnenblick EH, Ross J, Jr., Covell JW, Spotnitz HM, Spiro D. The ultrastructure of the heart in systole and diastole. Chantes in sarcomere length. Circ Res. 1967;21:423–431. doi: 10.1161/01.res.21.4.423. [DOI] [PubMed] [Google Scholar]
- 3.LeGrice IJ, Takayama Y, Covell JW. Transverse shear along myocardial cleavage planes provides a mechanism for normal systolic wall thickening. Circ Res. 1995;77:182–193. doi: 10.1161/01.res.77.1.182. [DOI] [PubMed] [Google Scholar]
- 4.LeGrice IJ, Smaill BH, Chai LZ, Edgar SG, Gavin JB, Hunter PJ. Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am J Physiol. 1995;269:H571–H582. doi: 10.1152/ajpheart.1995.269.2.H571. [DOI] [PubMed] [Google Scholar]
- 5.Spotnitz HM, Spotnitz WD, Cottrell TS, Spiro D, Sonnenblick EH. Cellular basis for volume related wall thickness changes in the rat left ventricle. J Mol Cell Cardiol. 1974;6:317–331. doi: 10.1016/0022-2828(74)90074-1. [DOI] [PubMed] [Google Scholar]
- 6.Cheng A, Langer F, Nguyen TC, Malinowski M, Ennis DB, Daughters GT, Ingels NB, Jr., Miller DC. Transmural left ventricular shear strain alterations adjacent to and remote from infarcted myocardium. J Heart Valve Dis. 2006;15:209–218. [PubMed] [Google Scholar]
- 7.Cheng A, Langer F, Rodriguez F, Criscione JC, Daughters GT, Miller DC, Ingels NB., Jr Transmural sheet strains in the lateral wall of the ovine left ventricle. Am J Physiol Heart Circ Physiol. 2005;289:H1234–H1241. doi: 10.1152/ajpheart.00119.2005. [DOI] [PubMed] [Google Scholar]
- 8.Cheng A, Langer F, Rodriguez F, Criscione JC, Daughters GT, Miller DC, Ingels NB., Jr Transmural cardiac strains in the lateral wall of the ovine left ventricle. Am J Physiol Heart Circ Physiol. 2005;288:H1546–H1556. doi: 10.1152/ajpheart.00716.2004. [DOI] [PubMed] [Google Scholar]
- 9.Harrington KB, Rodriguez F, Cheng A, Langer F, Ashikaga H, Daughters GT, Criscione JC, Ingels NB, Miller DC. Direct measurement of transmural laminar architecture in the anterolateral wall of the ovine left ventricle: new implications for wall thickening mechanics. Am J Physiol Heart Circ Physiol. 2005;288:H1324–H1330. doi: 10.1152/ajpheart.00813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez F, Langer F, Harrington KB, Cheng A, Daughters GT, Criscione JC, Ingels NB, Miller DC. Alterations in transmural strains adjacent to ischemic myocardium during acute midcircumflex occlusion. J Thorac Cardiovasc Surg. 2005;129:791–803. doi: 10.1016/j.jtcvs.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Gould KL, Kennedy JW, Frimer M, Pollack GH, Dodge HT. Analysis of wall dynamics and directional components of left ventricular contraction in man. Am J Cardiol. 1976;38:322–331. doi: 10.1016/0002-9149(76)90174-0. [DOI] [PubMed] [Google Scholar]
- 12.Dumesnil JG, Shoucri RM. Quantitative relationships between left ventricular ejection and wall thickening and geometry. J Appl Physiol. 1991;70:48–54. doi: 10.1152/jappl.1991.70.1.48. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher KP, Gerren RA, Stirling MC, Choy M, Dysko RC, McManimon SP, Dunham WR. The distribution of functional impairment across the lateral border of acutely ischemic myocardium. Circ Res. 1986;58:570–583. doi: 10.1161/01.res.58.4.570. [DOI] [PubMed] [Google Scholar]
- 14.Arts T, Reneman RS, Veenstra PC. A model of the mechanics of the left ventricle. Ann Biomed Eng. 1979;7:299–318. doi: 10.1007/BF02364118. [DOI] [PubMed] [Google Scholar]
- 15.Dou J, Tseng WY, Reese TG, Wedeen VJ. Combined diffusion and strain MRI reveals structure and function of human myocardial laminar sheets in vivo. Magn Reson Med. 2003;50:107–113. doi: 10.1002/mrm.10482. [DOI] [PubMed] [Google Scholar]
- 16.Takayama Y, Costa KD, Covell JW. Contribution of laminar myofiber architecture to load-dependent changes in mechanics of LV myocardium. Am J Physiol Heart Circ Physiol. 2002;282:H1510–H1520. doi: 10.1152/ajpheart.00261.2001. [DOI] [PubMed] [Google Scholar]
- 17.Weis SM, Emery JL, Becker KD, McBride DJ, Jr., Omens JH, McCulloch AD. Myocardial mechanics and collagen structure in the osteogenesis imperfecta murine (oim) Circ Res. 2000;87:663–669. doi: 10.1161/01.res.87.8.663. [DOI] [PubMed] [Google Scholar]
- 18.Knisley SB, Baynham TC. Line stimulation parallel to myofibers enhances regional uniformity of transmembrane voltage changes in rabbit hearts. Circ Res. 1997;81:229–241. doi: 10.1161/01.res.81.2.229. [DOI] [PubMed] [Google Scholar]
- 19.Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH. Left ventricular fibre architecture in man. Br Heart J. 1981;45:248–263. doi: 10.1136/hrt.45.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Liu W, Zhang H, Lacy L, Yang X, Song SK, Wickline SA, Yu X. Regional ventricular wall thickening reflects changes in cardiac fiber and sheet structure during contraction: quantification with diffusion tensor MRI. Am J Physiol Heart Circ Physiol. 2005;289:H1898–H1907. doi: 10.1152/ajpheart.00041.2005. [DOI] [PubMed] [Google Scholar]
- 21.Rohmer D, Siteck A, Gullberg GT. Reconstruction and visualization of fiber and laminar structures in the normal human heart from ex vivo diffuse tensor magnetic resonance imaging (DTMRI) data. Invest Radiol. 2007 Nov;42(11):777–89. doi: 10.1097/RLI.0b013e3181238330. [DOI] [PubMed] [Google Scholar]
- 22.Weber KT, Sun Y, Tyagi SC, Cleutjens JP. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol. 1994;26:279–292. doi: 10.1006/jmcc.1994.1036. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Song SK, Liu W, McLean M, Allen JS, Tan J, Wickline SA, Yu X. Remodeling of cardiac fiber structure after infarction in rats quantified with diffusion tensor MRI. Am J Physiol Heart Circ Physiol. 2003;285:H946–H954. doi: 10.1152/ajpheart.00889.2002. [DOI] [PubMed] [Google Scholar]
- 24.Kang PM, Izumo S. Apoptosis and heart failure: A critical review of the literature. Circ Res. 2000;86:1107–1113. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- 25.Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997;95:320–323. doi: 10.1161/01.cir.95.2.320. [DOI] [PubMed] [Google Scholar]
- 26.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 27.Tyagi SC, Lewis K, Pikes D, Marcello A, Mujumdar VS, Smiley LM, Moore CK. Stretch-induced membrane type matrix metalloproteinase and tissue plasminogen activator in cardiac fibroblast cells. J Cell Physiol. 1998;176:374–382. doi: 10.1002/(SICI)1097-4652(199808)176:2<374::AID-JCP16>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, John-Sutton MG, Gorman JH, III, Edmunds LH, Jr., Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation. 2003;107:2857–2863. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 29.Bowen FW, Jones SC, Narula N, John Sutton MG, Plappert T, Edmunds LH, Jr., Dixon IM. Restraining acute infarct expansion decreases collagenase activity in borderzone myocardium. Ann Thorac Surg. 2001;72:1950–1956. doi: 10.1016/s0003-4975(01)03282-9. [DOI] [PubMed] [Google Scholar]
- 30.Rijcken J, Bovendeerd PH, Schoofs AJ, van Campen DH, Arts T. Optimization of cardiac fiber orientation for homogeneous fiber strain during ejection. Ann Biomed Eng. 1999;27:289–297. doi: 10.1114/1.147. [DOI] [PubMed] [Google Scholar]
- 31.Rijcken J, Arts T, Bovendeerd P, Schoofs B, van Campen D. Optimization of left ventricular fibre orientation of the normal heart for homogeneous sarcomere length during ejection. Eur J Morphol. 1996;34:39–46. doi: 10.1076/ejom.34.1.39.13154. [DOI] [PubMed] [Google Scholar]
- 32.Bovendeerd PH, Arts T, Huyghe JM, van Campen DH, Reneman RS. Dependence of local left ventricular wall mechanics on myocardial fiber orientation: a model study. J Biomech. 1992;25:1129–1140. doi: 10.1016/0021-9290(92)90069-d. [DOI] [PubMed] [Google Scholar]
- 33.Ashikaga H, Criscione JC, Omens JH, Covell JW, Ingels NB., Jr Transmural left ventricular mechanics underlying torsional recoil during relaxation. Am J Physiol Heart Circ Physiol. 2004;286:H640, H647. doi: 10.1152/ajpheart.00575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]