Abstract
AIM: To characterize the expression of members of the transforming growth factor-beta (TGF-β)/Smad/connective tissue growth factor (CTGF) signaling pathway in the tissue of benign biliary stricture, and to investigate the effect of TGF-β signaling pathway in the pathogenesis of benign biliary stricture.
METHODS: Paraffin embedded materials from 23 cases of benign biliary stricture were analyzed for members of the TGF-β/Smad/CTGF signaling pathway. TGF-β1, TβRI, TβRII, Smad4, Smad7 and CTGF protein were detected by immunohistochemical strepto-advidinbiotin complex method, and CTGF mRNA was evaluated by hybridization in situ, while 6 cases of normal bile duct served as controls. The percentages of positive cells were counted. The correlation between TGF-β1, Smad4 and CTGF was analyzed.
RESULTS: The positive expression ratios of TGF-β1, TβRI, TβRII, Smad4, CTGF and CTGF mRNA in 23 cases with benign biliary stricture were 91.3%, 82.6%, 87.0%, 78.3%, 82.6% and 65.2%, respectively, significantly higher than that in 6 cases of normal bile duct respectively (vs 33.3%, 16.7%, 50.0%, 33.3%, 50.0%, 16.7%, respectively, P < 0.05). The positive expression ratio of Smad7 in cases with benign biliary stricture was 70.0%, higher than that in normal bile duct, but this difference is not statistically significant 70.0% vs 50%, P > 0.05). There was a positive correlation between positive expression of TGF-β1, Smad4 and CTGF in cases with benign biliary stricture.
CONCLUSION: The high expression of TGF-β/Smad/CTGF signaling pathway plays an important role in the pathogenesis of benign biliary stricture.
Keywords: Biliary stricture, Transforming growth factor-beta 1, Smad, Connective tissue growth factor, TβR
INTRODUCTION
Benign biliary strictures are caused by a heterogeneous group of benign conditions. They are usually iatrogenic, most frequently as a result of cholecystectomy[1]. The incidence of this complication has increased with the widespread use of laparoscopic cholecystectomy (LC)[2-4]. Other causes of benign biliary strictures include hepatolithiasis, recurrent cholangitis, infection with the fluke, Mirizzi syndrome, etc[5]. Anastomotic strictures are seen following bile duct reconstruction or orthotopic liver transplant (OLT)[6,7].
The main manifestations of benign biliary stricture are scar contracture and stenosis of bile duct, especially at the hepatic hilum or above[8]. The diagnosis and treatment of benign biliary strictures remains a clinical challenge, requiring a multidisciplinary approach. The pathogenesis of benign biliary stricture is still unclear.
It is well known that the cytokine transforming growth factor beta 1 (TGF-β1) has a key role either in the wound healing process or induction of fibrosis. The biological effect of TGF-β1 is regulated by a special signal transduction pathway[9,10]. Following activation of the TGF-β receptor, intracellular signal transduction is mediated by a variety of Smad proteins[11-13]. Connective tissue growth factor (CTGF), which promotes cell proliferation and deposition of extracellular matrix (ECM), is a downstream medium in the process in which TGF-β1 produces a marked effect on connective tissue cell. The aim of the present study was to determine the expressions of various cytokines in TGF-β/Smad/CTGF signaling pathway in tissue of benign biliary stricture, and to further investigate the role of TGF-β signaling pathway in the pathogenesis of benign biliary stricture.
MATERIALS AND METHODS
Patients and pathologic specimens
The study population included 23 patients (10 males and 13 females; mean age 50 years) who underwent benign biliary strictures (between June 2003 and November 2005, in Department of Hepatobiliary Surgery, First Affiliated Hospital, Medical College, Xi’an Jiaotong University, Xi’an). The average time interval between the first surgery and the second surgery was 16 mo, and the average admission time was 22 d. The causes of benign biliary stricture were bile duct injury or anastomotic stricture after biliary reconstruction (n = 17), hepato-lithiasis (n = 3) and recurrent cholangitis (n = 3). The specimens were obtained from the cicatrices of bile duct. Fibrosis was seen from HE staining. Six normal specimens of bile duct were obtained from donators in liver transplantation. The study protocol was approved by the Ethics Communittee of the First Affiliated Hospital, Medical College, Xi’an Jiaotong University.
Antibodies
Antibodies to TGF-β1 (Rabbit anti-human monoclonal antibody, Santa-dilution 1:400), TβRI (Rabbit anti-human monoclonal antibody, Santa-dilution 1:400), TβRII (Rabbit anti-human monoclonal antibody, Santa-dilution 1:300), Smad4 (Rabbit anti-human monoclonal antibody, Santa-dilution 1:400), Smad7 (Rabbit anti-human monoclonal antibody, Santa-dilution 1:400) and CTGF (Rabbit anti-human monoclonal antibody, Santa-dilution 1:300) were purchased from Wuhan Boster Biological Technology Co. Ltd.
Immunohistochemical analysis
The expression of TGF-β1, TβRI, TβRII, Smad4, Smad7 and CTGF was detected by SABC immunohisto-chemical method. The test kit of SABC was the product of Wuhan Boster Biological Technology Co. Ltd. In the control group, the primary antibody was replaced with PBS or normal rabbit serum. All paraffin embedded sections were deparaffinized and rehydrated, and pretreated for 20 min at 75°C in a microwave oven. After being treated with 1 mL/L H2O2 for 30 min to block the endogenous peroxidase, the sections were incubated with 20 mL/L fetal calf serum for 30 min to reduce nonspecific binding. Then the primary TGF-β1, TβRI, TβRII, Smad4, Smad7 and CTGF antibodies were applied to the sections and incubated at 4°C overnight. The sections were subsequently incubated with goat anti rabbit IgG at 37°C for 30 min, followed by incubation with SABC at 37°C for 30 min, and stained with DAB-H2O2 for 5-10 min and counterstained with hematoxylin.
In situ hybridization for CTGF mRNA
CTGF mRNA ISH detection kit and antisense oligonucleotide probe (digoxin-labeled) were purchased from Wuhan Boster Biological Technology Co. Ltd. A 30-mer sequence that is complementary to the region of CTGF mRNA was synthesized as follows: (1) 5'-CTG CTGCCGCGTCTGCGCCAAGCAGCTGGG-3'; (2) 5'-CAACTGCCTGGTCCAGACCACAGAGTGGAG-3'; (3) 5'-TGTACTACAGGAAGATGTACGGAGACATGG-3'. In brief, deparaffinized sections were incubated with 3% hydrogen peroxide for 30 min and then with 1 g/mL pepsin for 15 min. The prehybridization was performed at 37°C for 2 h, and the hybridization was conducted in a 42°C water bath for 18 h with each section covered with a soil coverslip. After thorough washing, tissue sections were preblocked for 20 min with blocking solution. Then, mouse anti-digoxin antibody was added for 60 min at 37°C. After washed in PBS, the sections were visualized according to the manufacturer’s instructions. A negative control was prepared by using a hybridization solution without the probe.
Assessment of staining reactions
A positive reaction was detected as plasmatic stain presenting in yellow or brown-yellow color. A modified Shimizu’s method[14] was used to assess staining reactions. We selected 10 visual fields under HP microscope and counted 100 cells randomly. The following scoring system was used: (1) score 0, positively stained cytoplasm in less than 5% of cells; score 1, 5% - 35% positive; score 2, 36%-65% positive; and score 3, > 66% positive; (2) The staining intensity was estimated using a 4-grade scoring system (0, 1, 2, 3): very weak (1+ staining in some cells) (Score 0); weak (1+ staining in cells) (Score 1); moderate (2+ staining in cells) (Score 2); strong (3+ staining in cells) (Score 3). The examiners were blinded to patients’ clinical and histological (HE staining) profile. Two investigators evaluated the staining levels independently, after which any discordant evaluations were adjusted by connected microscopes and scored jointly. The 1st and 2nd score were added together and divided 2, then the mean was made the final score; 0, 1, 1.5-2, 2.5-3 were recorded as (-), (+), (++), (+++) respectively.
Statistical analysis
The percentages of positive cells according to the final score of TGF-β1, TβRI, TβRII, Smad4, Smad7, CTGF and CTGF mRNA were calculated. Fisher’s exact probability test was used to analyze the relationship between stricture group and normal control group. Spearman linear correlation analysis was used to analyze correlativity between TGF-β1, Smad4 and CTGF, respectively. P < 0.05 was considered statistically significant in difference. Statistical analyses were performed with SPSS version 11.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Expression of TGF-β1, TβRI, TβRII Smad4, Smad7, CTGF and CTGF mRNA in benign biliary stricture group and normal control group
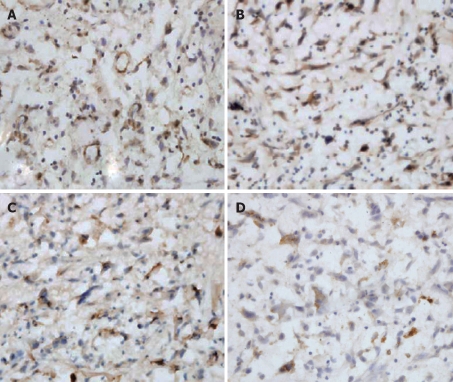
TGF-β1/TβR was expressed focally or diffusely in granulation tissue and cytoplasm of fibroblasts, macrophages, vascular endothelial cells, and inflammatory cells (Figure 1A). The expression of TGF-β1 was weak in fibrous tissue of normal bile duct wall. The positive percentages of TGF-β1, TβRI and TβRII at benign biliary stricture group were 91.3% (21/23), 82.6% (19/23) and 87.0% (20/23), respectively. Smad4/Smad7 was mainly expressed in cytoplasm and nucleus of fibroblasts, and some was expressed in fibrocytes (Figure 1B). The positive percentages of Smad4 and Smad7 at benign biliary stricture group were 78.3% (18/23) and 70.0% (16/23), respectively. Positive cells of CTGF were stained as yellow or brownish yellow granules in cytoplasm at immunohistochemical staining and in situ hybridization staining (Figure 1C and D). CTGF was expressed mainly in cytoplasm of fibroblasts in benign biliary stricture group, while it was scarely expressed in fibrocytes in the wall of common bile duct of normal control group. The positive percentages of CTGF and CTGF mRNA of benign biliary stricture group were 82.6% (19/23) and 65.2% (15/23), respectively. The percentages of positive expression of various cytokines in TGF-β1/Smad/CTGF signaling pathway in benign biliary stricture group and normal control group are shown in Table 1.
Figure 1.
A: TGF-β1 staining at stenotic bile duct (SABC, × 400); B: Smad4 staining at stenotic bile duct (SABC, × 400); C: CTGF staining at stenotic bile duct (SABC, × 400); D: CTGF mRNA staining at stenotic bile duct (INS, × 400).
Table 1.
Positive expression of various cytokines in TGF-β1/Smad/CTGF signaling pathway in benign biliary stricture group and normal control group
| Group | TGF-β1 | TβRI | TβRII | Smad4 | Smad7 | CTGF | CTGF mRNA |
| Benign biliary stricture (%) | 91.3a | 82.6a | 87.0a | 78.3a | 70.0 | 82.6a | 65.2a |
| Normal control (%) | 33.3 | 16.7 | 50.0 | 33.3 | 50.0 | 50.0 | 16.7 |
P < 0.05 vs control.
Linear correlation of TGF-β/ Smad/ CTGF expression
By Spearman linear correlation analysis, expression of TGF-β1 has positive correlation with expression of Smad4 (r = 0.848, P = 0.000) and CTGF (r = 0.848, P = 0.000); furthermore, Smad4 also has positive correlation with CTGF (r = 0.764, P = 0.000). Linear correlations of TGF-β/Smad/CTGF expression are shown in Figure 2.
Figure 2.
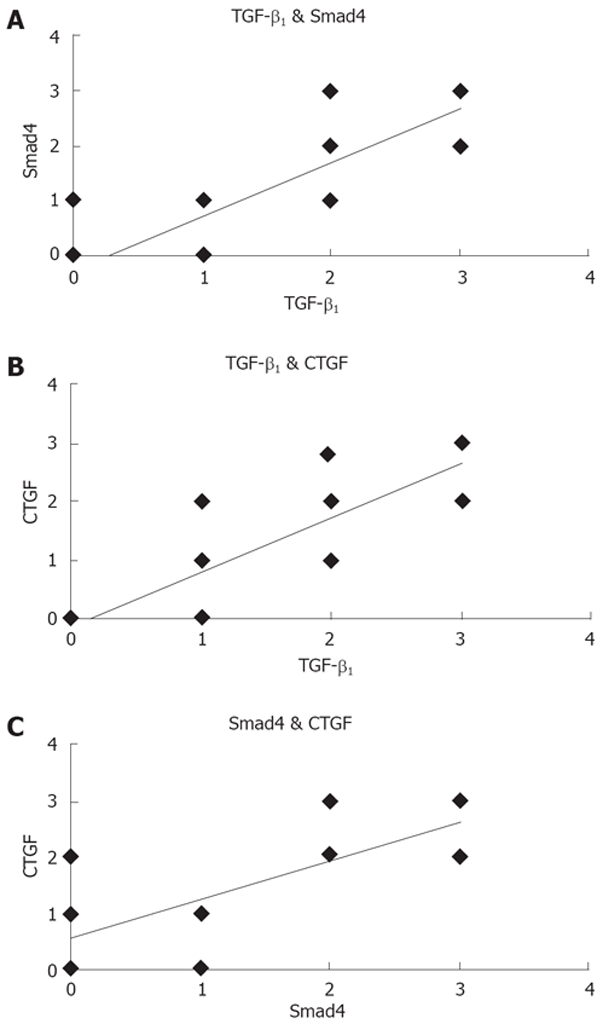
A: Correlation between TGF-β1 and Smad4; B: Correlation between TGF-β1 and CTGF; C: Correlation between Smad4 and CTGF.
DISCUSSION
In our previous study, we found that epithelial cells of the bile duct recovered poorly, chronic inflammation existed continuously, fibroblasts proliferated actively, collagens were over-deposited in submucosa, and reconstruction was poor after injury of bile duct. All these result in proliferation of cicatrix and high morbidity of stenosis of anastomosis[8]. Many studies have shown that TGF-β1 is the most important cytokine in the pathogenesis of over proliferation of cicatrix[15-17]. High expression of TGF-β1 and its receptors was found in keloid, and the expression of TGF-β1 was significantly high in fibroblasts of hypertrophic scar tissues that were cultivated in vitro[18]. In the present study, we observed that the expression of TGF-β1 in stenotic bile duct is significantly higher than that in normal bile duct, further confirming the outcome of our previous animal experiment[19].
Biological effects of TGF-β are regulated by specific signal transduction pathway. Smad family proteins have been identified as signal transducers for the TGF-β superfamily[20-22]. Following activation of the TGF-β receptor, intracellular signal transduction is mediated by a variety of Smad proteins. TGF-β1/Smad signal transduction pathway can regulate itself by positive and negative feedback regulation loops[23,24]. Smad4/Smad7 are the important factors in the two circuit loops, respectively. In the TβRI, TβRII and Smad signaling pathway, TGF-β1 can transfer and amplify signal, initiating its diverse cellular responses by binding to and activating specific cell surface receptors that have intrinsic serine/threonine kinase activity. These activated TGF-β receptors stimulate the phosphorylation of receptor-regulated Smad proteins (PSmad), which in turn form complexes with Smad4 that accumulate in the nucleus, activate the promoter of TGF-β1 and induce the expression of endogenous TGF-β1 by itself; this forms the positive feedback regulation loop. Meanwhile, PSmad proteins also can activate the promoter of Smad7 that inhibits TGF-β-induced transcriptional responses[25]. Psmad2 and Psmad3 may also affect its own promoter region, inhibiting transcription itself, and down-regulate the expression of R-Smad proteins, inhibiting signal transduction[26]. Accordingly, TGF-β1 inhibits its signal transduction by negative feedback regulation loop made by activating Smad7 expression and down-regulating R-Smad expression.
This study indicates that TβRI, TβRII, Smad4 in tissue of stenotic bile duct also have high expression besides TGF-β1; furthermore high, expression of TGF-β1 has positive correlation with Smad4 (r = 0.848, P = 0.000). We believe that this may be because high-expression of TGF-β can promote the expression of itself and its receptor by a positive feedback regulation loop. This over-expression then could amplify bioactivity and constantly activate smads complexes, which accumulate in the nucleus and regulate the transcription of target genes, resulting in active proliferation of fibroblasts and excessive collagen deposition. So, we can presume that high-expression of R-Smad and Co-smad maybe an important mechanism in pathogenesis of benign biliary stricture. Lasting stimulus of TGF-β1 that was constantly secreted in tissue of stenotic bile duct and high-sensitivity of the receptor to TGF-β1 possibly result in the above consequences.
This study also has observed that the expression of Smad7 is higher in tissue of stenotic bile duct than that in normal bile duct, but there was no statistical significance between the two groups. We think that because of the tissue difference, Smad7, which is up-regulated in benign biliary cicatrix does not predominate in the competition with R-smad that was also activated and up-regulated, so Smad7 can’t play a role in inhibitive function. R-smad, which predominates in the competition, is phosphorylated by TβRI and further binds to Smad4; the complex transfers into nucleus and regulates the expression of target genes.
CTGF is considered a modulator and assisted mediated factor that mediates biologic function of other molecules, and promotes fission of fibroblasts and accumulation of collagen[27]. CTGF can promote the synthesis of ECM such as collagen I, collagen III and FN. Lasky et al observed that CTGF is over-expressed in pathogenesis of fibrosis in skin, kidney, liver and heart[28]. Igarashi et al[29] thought that the expression of CTGF gene is directly regulated by TGF-β1. Grotendorst et al[30] also believed that CTGF, which promoted cell proliferation and deposition of ECM, was a downstream medium in the process in which TGF-β1 produced a marked effect on connective tissue cell.
The results have shown that expression of CTGF in stenotic bile duct tissue is significantly higher than in normal bile duct specimens. This difference indicated that CTGF plays an important role in the pathogenesis of over proliferation of cicatrix and benign biliary stricture. The expression of TGF-β1 has a positive correlation with that of CTGF (r = 0.848, P = 0.000); this confirms that both of them play roles of superior and inferior grade in signal transduction pathway in the pathogenesis of benign biliary stricture, similar to other fibrotic disease, whereas the positive correlation between expression of Smad4 and CTGF (r = 0.764, P = 0.000) indicates that the relationship between TGF-β1 and CTGF is connected by Smads signaling pathway.
Taken together, the present study indicates that the mechanism of effect in TGF-β1/Smad/CTGF signaling pathway is as follows. The continuity of bile duct wall is destroyed after bile duct injury, which results in changes of adjacent intra-cellular metabolism. Then, TGF-β is released and binded to the type II receptor at first; thus a binary complex is formed. Type I receptor then is recruited and phosphorylated in its GS domain by TβRII, leading to activation of its kinase activity and subsequent formation of a signaling complex. R-Smads are phosphorylated by this signaling complex, and in turn can form heteromeric complexes with Smad4. These activated Smad complexes accumulate in the nucleus, where they directly or indirectly bind to specific promoter regions on target genes together with transcription factor (TF) and/or co-activators/repressors, and downstream mediator CTGF is activated. The activation of TGF-β/Smad/CTGF signal transduction pathway can result in fibrosis in the interaction between cell and ECM, cause disorder of metabolism and regulation between inflammatory cell, repairing cell and collagen. Fibrocytes in submucosa are transformed into activated fibroblasts, which proliferate abundantly, and synthesize and secrete collagen fibers. Under the stimulus of bile and secondary infection, inflammation extends and the wound healing process disorders, chronic inflammation exists continuously, fibroblasts proliferate constantly, collagens are over-deposited in submucosa. All these result in a prolonged healing process of bile duct, proliferation of cicatrix and morbidity of benign biliary stricture.
COMMENTS
Background
Transforming growth factor beta 1 (TGF-β1) has a key role either in the wound healing process or induction of fibrosis. The biological effects of TGF-β1 are regulated by TGF-β/Smad/connective tissue growth factor (CTGF) signaling pathway. The main manifestations of benign biliary stricture are scar contracture and stenosis of bile duct. The exact role of TGF-β signaling pathway in the pathogenesis of benign biliary stricture is still unknown.
Research frontiers
In the last few years, many studies have shown that TGF-β1 is the most important cytokine in the pathogenesis of over proliferation of cicatrix. Biological effects of TGF-β are regulated by specific signal transduction pathway. Smad family proteins have been identified as signal transducers for the TGF-β superfamily. The expression of CTGF gene is regulated directly by TGF-β1. CTGF, which promotes cell proliferation and deposition of extracellular matrix (ECM), is a downstream medium in the process in which TGF-β1 produces a marked effect on connective tissue cell.
Innovations and breakthroughs
The high expression of TGF-β/Smad/CTGF signaling pathway plays an important role in the pathogenesis of benign biliary stricture. These results demonstrate a new view of TGF-β signaling pathway involved in the benign biliary stricture.
Applications
This study indicates that the mechanism of TGF-β1/Smad/CTGF signaling pathway in pathogenesis of benign biliary stricture. It may provide new targets for further understanding of the pathogenesis of benign biliary stricture and new therapeutic targets.
Terminology
Smads are the only substrates for type I receptor kinases known to have a signalling function; they were first identified as the products of the Drosophila Mad and C. elegans Sma genes, which lie downstream of the BMP-analogous ligand-receptor systems in these organisms. Smad proteins transduce signals from TGF-β superfamily ligands that regulate cell proliferation, differentiation and death through activation of receptor serine/threonine kinases.
Peer review
The study is well designed and contains important information and novelties concerting TGF-β1 signaling pathway in benign billiary stricture. The authors performed a great amount of experiments and a comprehensive statistical analysis of data.
Footnotes
Supported by The grant from Shaanxi Science and Technology Project, No. 2002K10-G8
Peer reviewer: Ana Cristina Simões e Silva, Professor, Pediatrics Department, Federal University of Minas Gerais Institution, Avenida Professor Alfredo Balena, 190, Belo Horizonte 30130-100, Brazil
S- Editor Li DL L- Editor Li M E- Editor Lin YP
References
- 1.Larghi A, Tringali A, Lecca PG, Giordano M, Costamagna G. Management of hilar biliary strictures. Am J Gastroenterol. 2008;103:458–473. doi: 10.1111/j.1572-0241.2007.01645.x. [DOI] [PubMed] [Google Scholar]
- 2.Adamsen S, Hansen OH, Funch-Jensen P, Schulze S, Stage JG, Wara P. Bile duct injury during laparoscopic cholecystectomy: a prospective nationwide series. J Am Coll Surg. 1997;184:571–578. [PubMed] [Google Scholar]
- 3.Connor S, Garden OJ. Bile duct injury in the era of laparoscopic cholecystectomy. Br J Surg. 2006;93:158–168. doi: 10.1002/bjs.5266. [DOI] [PubMed] [Google Scholar]
- 4.McKenzie S, Schwartz R. The management of bile duct injuries occurring during laparoscopic cholecystectomy. Curr Surg. 2006;63:20–23. doi: 10.1016/j.cursur.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Judah JR, Draganov PV. Endoscopic therapy of benign biliary strictures. World J Gastroenterol. 2007;13:3531–3539. doi: 10.3748/wjg.v13.i26.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin RF, Rossi RL. Bile duct injuries. Spectrum, mechanisms of injury, and their prevention. Surg Clin North Am. 1994;74:781–803; discussion 805-807. [PubMed] [Google Scholar]
- 7.Porayko MK, Kondo M, Steers JL. Liver transplantation: late complications of the biliary tract and their management. Semin Liver Dis. 1995;15:139–155. doi: 10.1055/s-2007-1007271. [DOI] [PubMed] [Google Scholar]
- 8.Geng ZM, Yao YM, Liu QG, Niu XJ, Liu XG. Mechanism of benign biliary stricture: a morphological and immunohistochemical study. World J Gastroenterol. 2005;11:293–295. doi: 10.3748/wjg.v11.i2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultze-Mosgau S, Blaese MA, Grabenbauer G, Wehrhan F, Kopp J, Amann K, Rodemann HP, Rodel F. Smad-3 and Smad-7 expression following anti-transforming growth factor beta 1 (TGFbeta1)-treatment in irradiated rat tissue. Radiother Oncol. 2004;70:249–259. doi: 10.1016/j.radonc.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Wu XY, Yang YM, Guo H, Chang Y. The role of connective tissue growth factor, transforming growth factor β1 and Smad signaling pathway in cornea wound healing. Chin Med J. 2006;119:57–62. [PubMed] [Google Scholar]
- 11.Ashcroft GS, Roberts AB. Loss of Smad3 modulates wound healing. Cytokine Growth Factor Rev. 2000;11:125–131. doi: 10.1016/s1359-6101(99)00036-2. [DOI] [PubMed] [Google Scholar]
- 12.Attisano L, Silvestri C, Izzi L, Labbe E. The transcriptional role of Smads and FAST (FoxH1) in TGFbeta and activin signalling. Mol Cell Endocrinol. 2001;180:3–11. doi: 10.1016/s0303-7207(01)00524-x. [DOI] [PubMed] [Google Scholar]
- 13.Bitzer M, von Gersdorff G, Liang D, Dominguez-Rosales A, Beg AA, Rojkind M, et al. A mechanism of suppression of TGF-beta/SMAD signaling by NF-kappa B/RelA. Genes Dev. 2000;14:187–197. [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu M, Saitoh Y, Itoh H. Immunohistochemical staining of Ha-ras oncogene product in normal, benign, and malignant human pancreatic tissues. Hum Pathol. 1990;21:607–612. doi: 10.1016/s0046-8177(96)90006-4. [DOI] [PubMed] [Google Scholar]
- 15.Chin D, Boyle GM, Parsons PG, Coman WB. What is transforming growth factor-beta (TGF-beta)? Br J Plast Surg. 2004;57:215–221. doi: 10.1016/j.bjps.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Beanes SR, Dang C, Soo C, Ting K. Skin repair and scar formation: the central role of TGF-beta. Expert Rev Mol Med. 2003;5:1–22. doi: 10.1017/S1462399403005817. [DOI] [PubMed] [Google Scholar]
- 17.Robson MC. Proliferative scarring. Surg Clin North Am. 2003;83:557–569. doi: 10.1016/S0039-6109(02)00197-4. [DOI] [PubMed] [Google Scholar]
- 18.Wang R, Ghahary A, Shen Q, Scott PG, Roy K, Tredget EE. Hypertrophic scar tissues and fibroblasts produce more transforming growth factor-beta1 mRNA and protein than normal skin and cells. Wound Repair Regen. 2000;8:128–137. doi: 10.1046/j.1524-475x.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- 19.Geng ZM, Xiang GA, Han Q, Liu XG, Liu QG, Pan CE. The expression and significance of macrophage and TGF-β1 on healing process of bile duct. Zhonghua Shiyan Waike Zazhi. 2000;17:522–523. [Google Scholar]
- 20.Xu L. Regulation of Smad activities. Biochim Biophys Acta. 2006;1759:503–513. doi: 10.1016/j.bbaexp.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuse Y, Hashimoto N, Maekawa M, Toyama Y, Nakao A, Iwamoto I, Sakurai K, Suzuki Y, Yagui K, Yuasa S, et al. Activation of the Smad pathway in glomeruli from a spontaneously diabetic rat model, OLETF rats. Nephron Exp Nephrol. 2004;98:e100–e108. doi: 10.1159/000080685. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Fu X, Sheng Z. Review of current progress in the structure and function of Smad proteins. Chin Med J (Engl) 2002;115:446–450. [PubMed] [Google Scholar]
- 23.ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakao A, Okumura K, Ogawa H. Smad7: a new key player in TGF-beta-associated disease. Trends Mol Med. 2002;8:361–363. doi: 10.1016/s1471-4914(02)02376-6. [DOI] [PubMed] [Google Scholar]
- 26.Mori Y, Chen SJ, Varga J. Modulation of endogenous Smad expression in normal skin fibroblasts by transforming growth factor-beta. Exp Cell Res. 2000;258:374–383. doi: 10.1006/excr.2000.4930. [DOI] [PubMed] [Google Scholar]
- 27.Leask A, Abraham DJ. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003;81:355–363. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- 28.Lasky JA, Ortiz LA, Tonthat B, Hoyle GW, Corti M, Athas G, Lungarella G, Brody A, Friedman M. Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am J Physiol. 1998;275:L365–L371. doi: 10.1152/ajplung.1998.275.2.L365. [DOI] [PubMed] [Google Scholar]
- 29.Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993;4:637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–480. [PubMed] [Google Scholar]