Abstract
This year marks the twenty-fifth anniversary of the first Annual Review in Immunology article to describe features of the T cell antigen receptor (TCR). In celebration of this anniversary, we begin with a brief introduction outlining the chronology of the earliest studies that established the basic paradigm for how the engaged TCR transduces its signals. This review continues with a description of the current state of our understanding of TCR signaling, as well as a summary of recent findings examining other key aspects of T cell activation including crosstalk between the TCR and integrins, the role of costimulatory molecules, and how signals may negatively regulate T cell function.
Keywords: signal transduction, immunoreceptor, integrin
Introduction
Twenty-five years ago, Annual Review in Immunology published its first review describing features of the structure we now know as the T cell antigen receptor (TCR) (1). In recognition of this anniversary, this article begins by highlighting a sampling of seminal observations made in the decade following the initial description of the TCR. The discoveries made during this period established the basic paradigm for how TCR engagement initiates the earliest biochemical events leading to cellular activation, described nicely in an Annual Review in Immunology article in 1996 (2) (Figure 1a). This historical perspective sets the stage for a discussion of our current state of understanding of the molecular and biochemical events critical for T cell activation that have emerged from the work of multiple laboratories.
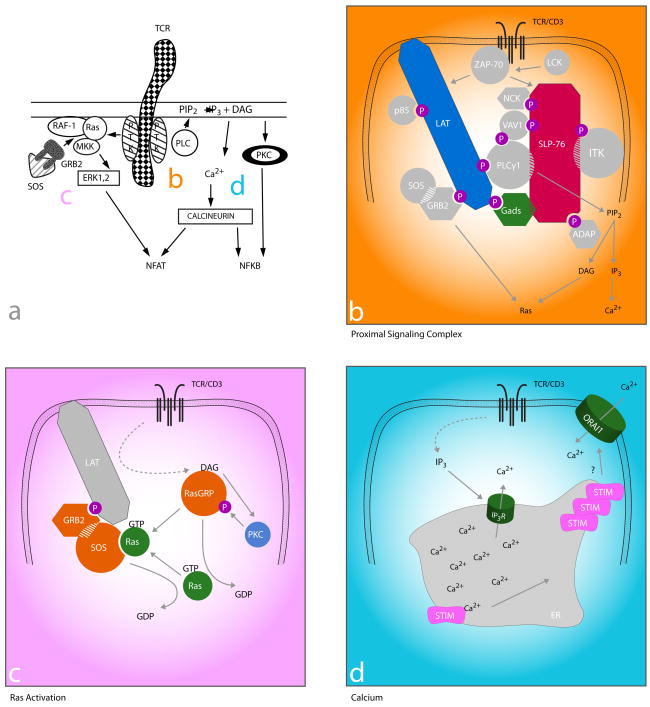
Figure 1. TCR proximal signaling then and now: filling in the gaps.
Then: a. TCR signaling mid-1990s (adapted from 2). Ligation of the TCR/CD3 results in activation of src and syk family PTKs associated with the intracellular CD3 domains that then activate PLC and Ras-dependent pathways.
Now: b–d. Current understanding of how the TCR couples to PLC activation (b), Ras (c), and the molecular basis for Ca2+ influx (d). b. The link between PTKs and downstream pathways (including those in c and d) is a multi-molecular signaling complex nucleated by the adapter proteins LAT, Gads, and SLP-76. Lck activates ZAP-70 to phosphorylate (p) tyrosine residues on LAT, which then recruits Gads and its constitutive binding partner SLP-76. ZAP-70-mediated phosphorylation of SLP-76 results in the recruitment of multiple SH2 domain-containing effector molecules (circles) and adapter proteins (octogons). SH3 domains (hatching) also link effectors to adapters and contribute to stabilization of the complex. c. Activation of Ras involves two Ras GEFs (Sos and RasGRP) in a positive feedback loop. TCR-induced production of DAG (see b) results in the membrane recruitment of RasGRP, where it is phosphorylated and activated by PKC. RasGRP then facilitates the removal of GDP from Ras, which can then bind GTP and become activated. GTP-bound Ras then binds Sos (bound constitutively to GRB2, which is inducibly recruited to LAT), increasing its GEF activity resulting in a positive feedback loop and robust Ras activity. d. The link between depletion of ER Ca2+stores and CRAC channel activation is Stim. TCR-induced IP3 production (see b) results in the activation of IP3 receptors (IP3R), which release Ca2+ from the ER into the cytoplasm. Stim contains paired EF hands within the ER lumen, which bind Ca2+. Upon depletion of ER stores, Ca2+-free Stim molecules cluster and are thought to move to areas of ER/plasma membrane proximity where they co-localize with CRAC channels (the pore-forming subunit has been identified as Orai1) and contribute to their activation and the subsequent influx of Ca2+.
Describing the TCR Complex
In the early 1980s, several groups began experiments to identify and characterize the antigen receptor on T cells. One approach made use of newly developed molecular techniques, ultimately leading to the identification of the genes responsible for the antigen-binding proteins (3–5). An even earlier approach relied on immunization of mice with T cell clones of a defined specificity, hybridomas, or clonal T cell tumors in the hope that antibodies would be generated that would react with the receptor responsible for binding to antigen (6–8). Such antibodies were produced and were then used first to demonstrate interference with antigenic responses and later to perform the initial biochemical characterization of the receptor itself. These studies revealed a complicated cell surface structure that included proteins reactive with antibodies against the nonpolymorphic CD3 proteins (initially thought of as three polypeptides, γ, δ, and ε) (9), as well as variable proteins designated α and β.
The antigen-binding function of αβ was obvious early on, both by inference, due to their highly polymorphic nature and similarity to immunoglobulin, and experimentally, as early gene transfer experiments demonstrated that antigen/major histocompatibility complex (MHC) reactivity tracked with expression of these receptor components (10, 11). However, it was less obvious what role the CD3 proteins played in TCR function. Several lines of evidence suggested that the CD3 molecules were critical for signal transduction: unlike αβ, the CD3 molecules had long cytoplasmic tails, and anti-CD3 antibodies resulted in T cell activation, although it was difficult to conclusively demonstrate signaling function. One early effort involved an attempt to create cell lines expressing αβ without CD3 or CD3 without αβ, thereby creating reagents to investigate the independent role of the receptor components. These experiments were not successful, as it became clear that there is an obligatory co-expression of αβ with CD3 (12). These studies did result, however, in the creation of the first in a long line of genetically altered Jurkat T cells that have been instrumental in our understanding of how the TCR complex couples to its signaling machinery (13).
Early Signaling Studies
Initial investigations into how the TCR transduces its signals began with the observation that TCR-deficient Jurkat T cells could be stimulated pharmacologically with the combination of phorbol esters and Ca2+ ionophores (14). This observation led to speculation that engagement of the TCR might stimulate the same signals that these reagents induced. This notion was tested directly in experiments demonstrating increases in intracellular free Ca2+ following CD3 or TCR stimulation in both Jurkat cells and primary T cells. The source of the Ca2+ increase was shown to be a combination of Ca2+ released from an intracellular pool in response to inositol trisphosphate (IP3) production and influx of Ca2+ from outside of the cell (15). It was presumed that phorbol esters were important because of their ability to activate protein kinase C (PKC, now known to be a family of enzymes), whose activity is regulated physiologically by diacylglycerol (DAG). Concurrent studies in other systems had demonstrated that a single enzymatic reaction, hydrolysis of the membrane phospholipid phosphatidyl inositiol 4,5 bisphosphate by phospholipase C (PLC), generates both IP3 and DAG, suggesting that the TCR may function to regulate PLC activity.
Testing this notion proved somewhat difficult at first, as PLC regulation was initially described downstream of heterotrimeric guanosine triphosphate (GTP)-binding proteins associated with seven transmembrane domain receptors. An exhaustive but unsuccessful search ensued for the GTP binding protein critical for coupling the TCR to PLC activation. Insight into how the TCR may initiate PLC activation emerged from a confluence of discoveries in immune cells and other lineages. These discoveries included the finding that stimulation of the TCR resulted in changes in protein phosphorylation, including inducible phosphorylation of the newly described ζ chain of the CD3 complex (16) and the observation that growth factor receptors with intrinsic protein tyrosine kinase (PTK) activity also stimulated PLC function. In contrast to seven transmembrane domain receptors, however, receptor PTKs were shown to stimulate another PLC isoform, PLCγ. Although none of the TCR components possessed enzymatic activity themselves, cytosolic PTKs of the src family (in particular lck and fyn) were being described in T cells. These PTKs were either associated with the TCR (17) or with the CD4 and CD8 co-receptors (18, 19), both known to be important for TCR signaling. These observations, coupled with the new knowledge that T cell activation required PTK function (20), led to a new TCR signaling paradigm in which the TCR recruited cytosolic PTKs to activate key second messengers.
How CD3 Transduces Its Signals
Testing this model led to a search for additional substrates of the TCR-stimulated PTKs. Among the most attractive candidates were tyrosines within the CD3 molecules themselves that fell within a motif present once within CD3 ε, γ, and δ and in triplicate within each ζ chain and found also in key immunoreceptors on other immune cell lineages. The signature of these motifs (21), eventually designated immunoreceptor tyrosine-based activation motifs (ITAMs), is two tyrosines flanking a series of amino acids including key leucine/isoleucines with stereotypic spacing. Numerous laboratories demonstrated that the ITAM tyrosines were in fact phosphorylated upon TCR ligation, but defining the importance of this posttranslational modification for TCR function was not trivial. Since the CD3 molecules could not be expressed without the αβ chains, several groups created chimeric molecules fusing the cytoplasmic domains of individual CD3 chains to extracellular and transmembrane domains from other proteins (22–24). These cDNAs in chimeric proteins were then transfected into T cell lines selected for TCR loss. Antibody crosslinking of the extracellular domain of the chimeras recapitulated all of the known TCR-mediated signaling events leading to cellular activation. Mutation of the key ITAM tyrosines (or altering their spacing) abrogated the ability of the chimeras to activate the cells, thus demonstrating that tyrosine phosphorylation of the CD3 ITAMs was an early and requisite step for TCR-mediated T cell activation.
Studies of the CD3 chimeras led naturally to the question of the purpose for ITAM phosphorylation. Unlike growth factor receptors whose intrinsic enzymatic activity is enhanced by tyrosine phosphorylation, the CD3 molecules have no such effector function on their own. It was speculated, therefore, that tyrosine phosphorylation of the ITAMs might serve as docking sites for interactions with other proteins. Indeed, it was soon shown that phosphorylated CD3ζ (and later other ITAM-containing proteins) was a recruitment site of a 70 kDa phosphoprotein that turned out to be the syk kinase family member ZAP-70 (ζ-associated protein of 70 kDa) PTK (25). A model therefore emerged that engagement of the TCR led to src PTK activity resulting in ITAM phosphorylation and recruitment of ZAP-70. This converted the TCR with no intrinsic enzymatic function to an active PTK able to phosphorylate a spectrum of substrates leading to a myriad of downstream signals that, when integrated appropriately (along with signals from other co-receptors), leads to T cell activation (26).
The basic tenets of this model have stood the test of intensive investigation. In the 15 years since ZAP-70 was cloned, investigators have filled in many of the gaps between the TCR and initiation of effector functions. Much has been learned about substrates of the PTKs (including src, syk, and more recently tec family members) activated by the TCR and how these molecules participate in T cell activation, about how signaling complexes are organized by adapter proteins to bring effector proteins together, and about the unexpected intersection of particular signaling pathways. With the accumulation of data, it has also become clear that signaling via the TCR complex is not a linear event starting at the receptor and ending in the nucleus. Instead, there appears to be complex feedback and feedforward regulation at each step. Ironically, one of the most central signaling questions that remains is how does receptor binding translate most proximally into an activating signal. Many models have been proposed, but none has yet withstood the rigor of subsequent investigation. This review summarizes our current understanding of many of these issues and poses some of the intriguing questions that remain.
Initiating TCR Signal Transduction
Since identification of the TCR as a complex consisting of the variable αβ chains non-covalently associated with the non-polymorphic CD3 proteins, considerable work has gone into defining the stoichiometry of these interacting molecules. It is now known that the CD3 proteins exist as a series of dimers including γε, δε, and ζζ associated with a single αβ heterodimer. Although it has been clear for more than 15 years that CD3 transduces signals from the engaged receptor via its ITAMs, exactly how ligation of the TCR is translated into the first signal remains controversial. Current models suggest that both TCR aggregation and conformational changes may play roles in signal initiation.
Two separate but not mutually exclusive conformational changes within the CD3 cytoplasmic tails have been proposed as mechanisms for TCR-inducible ITAM phosphorylation. It was shown that in the presence of lipid, the cytoplasmic tail of CD3ζ folds in such a way as to prevent phosphorylation, while in aqueous solution, the CD3 tail loses this conformation and is able to be phosphorylated by lck (27). This finding led to the hypothesis that in resting T cells the CD3ζ tail is tightly associated with the lipid-rich inner leaflet of the plasma membrane, rendering it inaccessible to lck phosphorylation, but following TCR ligation, it is released from the membrane and phosphorylated. However, a rigorous test for this model in T cells has not yet been provided. More recently, attention has turned to a possible conformational change in CD3ε with the observation that upon TCR ligation a proline-rich region (PRR) in CD3ε is exposed and available to recruit an SH3 domain of the adapter protein non-catalytic tyrosine kinase (Nck). Importantly, this occurs prior to CD3ζ and CD3ε ITAM phosphorylation (28). It was speculated that Nck could then recruit and activate effector molecules required for subsequent ITAM phosphorylation. However, recent data using mice with retrogenic or knock-in mutations of the CD3ε PRR have failed to show a requirement for the CD3ε/Nck interaction in the activation of peripheral T cells (29, 30). This observation must be considered in light of the T cell developmental abnormalities in the knock-in mice. Resolving the importance of the PRR for mature T cell function requires temporal control of expression of the mutant.
Although the seminal finding that there is a non-ITAM region in CD3 that is critical for TCR signaling appears firm, several controversies remain regarding the CD3ε PRR. There is disagreement regarding whether the interaction between Nck and CD3ε is constitutive rather than inducible (29, 31–33). The role of the CD3ε/Nck interaction in mature T cells is also controversial, as it has been suggested that the interaction (i) is not required for mature T cell activation (30), (ii) is required for signal amplification and ITAM phosphorylation following weak TCR ligation (32), or (iii) is required for regulation of TCR activity by inhibiting ITAM phosphorylation and promoting TCR degradation (33). Ongoing work will provide insights into solving these controversies.
While CD3 conformational changes may offer explanations for the initiation of the intracellular kinase cascade, it remains to be answered how peptide/MHC (pMHC)/TCR interactions result in conformational changes in the intracellular regions of the CD3 complex. Models based on ligand-induced conformational changes in the TCR and/or TCR aggregation have been proposed (Table 1). The crystal structures of several pMHC/TCR complexes have revealed little conformational changes in the TCR heterodimer outside of the binding domain (reviewed in 34). However, due to the current technical limitations of crystallography and protein nuclear magnetic resonance spectroscopy, only pMHC/TCR structures in the absence of transmembrane domains and CD3 components have been elucidated. Therefore, changes between the components of the TCR/CD3 complex in relation to one another or to the plasma membrane cannot be ruled out. It has been proposed that the rigid, rod-like transmembrane domain of the CD3εγ could potentially mediate a piston-like movement of the TCR complex perpendicular to the plasma membrane after ligand binding, in a manner similar to that reported for the aspartate receptor (35). Such a model would require external forces that could potentially be provided by cell-to-cell contact, but this possibility is difficult to reconcile with the ability of soluble ligands to activate the TCR. Similarly, an external force is required for the recently proposed “receptor deformation” model, whereby the movement of the T cell as it travels across the antigen-presenting cell (APC) exerts a force on transient pMHC/TCR interactions. Only when the force required to induce conformational changes in the TCR is less than the force required to break the pMHC/TCR interaction will TCR triggering occur (36). Alternatively, the “permissive geometry” model for TCR triggering predicts that dimeric pMHCs bind two TCR/CD3 complexes (37). This interaction results in a rotation of the TCR heterodimers around one another, displacing the extracellular domains of the associated CD3 molecules. The transmembrane interactions between the CD3 and TCR dimers serve as pivot points, and the CD3 movement is scissor-like, exposing previously shielded ITAMs and/or other functionally important domains. This model is consistent with studies that suggest that TCR aggregation is required for TCR triggering.
Table 1.
Recent models for initial TCR triggering
| Model | Description |
|---|---|
| Piston-like movement | An external force from the APC/T cell interaction pushes the TCR/CD3 complex in a piston-like fashion perpendicular to the plasma membrane, exposing activating motifs on the intracellular regions of CD3 chains |
| Receptor-deformation | The force of the motility of the T cell as it moves over an APC induces TCR triggering only when pMHC/TCR interactions are of a high enough affinity to withstand the force long enough to undergo a conformational change |
| Permissive geometry | pMHC dimers bind TCR/CD3 dimers inducing a rotational scissor-like conformational change in CD3 chain(s) that reveal previously hidden intracellular activation motif(s) |
| Kinetic segregation | Signal initiation occurs due to exclusion of inhibitory molecules in the tight contact zone between the T cell and the APC, thereby shifting the enzymatic steady state towards an activating state |
| Diffusion trapping | Expanded kinetic segregation model in which the affinity/diffusion coefficient of the pMHC/TCR dictates the valency of TCRs required to “trap” the complex in the tight contact zone and therefore initiate a productive signal |
| Pseudodimer | Agonist pMHC/TCR recruits a second TCR via interaction with its associated CD4, the second TCR then binds a “co- agonist” endogenous pMHC forming a stable pseudodimer that triggers signaling via proximity of ITAMs to CD4- associated lck and/or by conformational change |
TCR aggregation has long been implicated as a mechanism for TCR triggering. In the classic aggregation model, cross-linking of TCR/CD3 complexes with multimeric pMHC enables close contact and transphosphorylation between the CD3 tails and associated PTKs. Clustering of several TCR complexes also may result in competition for membrane between the CD3ζ chains, resulting in dissociation of the ζ chains from the membrane (27). Aggregation is supported strongly by observations that soluble multimeric but not monomeric pMHC can trigger TCR activation. However, recent biochemical and microscopic studies suggest that preformed TCR aggregates are present on non-activated T cells (reviewed in 38). In the “kinetic segregation” model, adhesion and other accessory molecules with short extracellular domains such as CD2 initiate close contact zones between an APC and a T cell (39). Inhibitory phosphatases with long extracellular domains such as CD45 are excluded due to their size. TCR complexes that engage pMHC on the APC surface remain in the close contact zone, where they are segregated from phosphatases and are able to initiate signal. TCR complexes that do not engage pMHC are free to diffuse outside of the close contact zone. Shortening the extracellular domains of inhibitory molecules or lengthening the extracellular domains of adhesion or accessory molecules can abrogate TCR signaling, suggesting that kinetic segregation is an important aspect of TCR signal initiation (reviewed in 39). This notion was expanded with the “diffusion trapping” model in which immobilization or trapping of pMHC/TCRs in the close contact zones is responsible for TCR signal initiation (40). The valency, or degree of aggregation, of the pMHC/TCR required for trapping and therefore triggering is dependent on the affinity and diffusion coefficient of the pMHC/TCR interaction. Another model has been proposed that suggests that endogenous pMHCs amplify signals produced by the rare agonist pMHCs by promoting TCR aggregation and a subsequent phosphorylation cascade (38). Similarly, the pseudodimer model proposes that an agonist pMHC/TCR can recruit a second TCR in a CD4-dependent manner, and this second TCR binds a “co-agonist” endogenous pMHC forming a stable pseudodimer that could potentially trigger signaling through ITAM/lck proximity or possibly through CD3 conformational changes (41). Many of these models are not mutually exclusive, and it is likely that TCR aggregation, conformational changes within the TCR complex, and exclusion of inhibitory molecules are all required for TCR triggering, perhaps in a stepwise fashion.
Proximal Signaling Complex
The earliest step in intracellular signaling following TCR ligation is the activation of src (lck and fyn) PTKs leading to phosphorylation of the CD3 ITAMs. Recruitment of ZAP-70 follows, leading to a cascade of phosphorylation events. The past decade has seen the description of a subcellular assembly and activation of an adapter protein nucleated multi-molecular signaling complex (Figure 1b). This complex is responsible for propagating the TCR/PTK signal into multiple and diverse distal signaling pathways.
Among the most important of the ZAP-70 targets are the transmembrane adapter protein linker for the activation of T cells (LAT) and the cytosolic adapter protein src homology 2 (SH2) domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76) (42, 43). These two adapters form the backbone of the complex that organizes effector molecules in the correct spatio-temporal manner to allow for the activation of multiple signaling pathways. The importance of these adapters is underscored by studies showing that the loss of either LAT or SLP-76 results in a near complete loss of TCR signal transduction reminiscent of syk/ZAP-70 or lck/fyn doubly deficient T cells (44–46).
LAT contains nine tyrosines that are phosphorylated upon TCR engagement, which have been shown to bind the C-terminal SH2 domain of PLCγ1, the p85 subunit of phosphoinositide 3-kinase (PI3K), and the adapters growth factor receptor-bound protein 2 (GRB2) and GRB2-related adapter downstream of Shc (Gads) (reviewed in 45). SLP-76 is then recruited to phosphorylated LAT via their mutual binding partner Gads (47). SLP-76 itself contains three modular domains: an N-terminal acidic domain with three phosphorylatable tyrosines that interact with the SH2 domains of Vav1, Nck, and IL-2-induced tyrosine kinase (Itk); a PRR that binds constitutively Gads and PLCγ1; and a C-terminal SH2 region that can bind adhesion and degranulation-promoting adapter protein (ADAP) and hematopoietic progenitor kinase 1 (HPK1) (reviewed in 44). While LAT and SLP-76 serve to nucleate this large signaling complex, the effector molecules themselves also are important for stabilizing the complex. For example, the Tec family kinase Itk is required in a kinase-independent manner for the recruitment of the guanine nucleotide exchange factor (GEF) Vav1 to the APC contact site, while Vav1 is required for optimal SLP-76 phosphorylation and recruitment to LAT as well as for Itk activation (48–50). These and other data suggest that the formation of the complex is more complicated than the linear model most often invoked for simplicity. For example, PLCγ1 has been shown to directly bind to SLP-76, LAT, Vav1, as well as its activating kinase Itk (reviewed in 51). It is thought that these interactions collectively are required to stabilize PLCγ1 in the correct conformation within the complex to allow for its optimal activity (52). Advancements in biochemical and structural techniques are needed to elucidate the precise allosteric and perhaps stochiometric changes within the multimolecular complex that allow for signal transduction.
To investigate more precisely the importance of these complex interactions in primary T cells, several laboratories have generated mice expressing transgenic or knock-in mutations in specific binding regions in various molecules involved in proximal signaling (53–55). Tyrosine to phenylalanine mutations in SLP-76 at residues 112 and 128 or 145 in primary thymocytes and T cells does not result in a loss of SLP-76/Vav1/Nck/Itk interactions, as would be expected from earlier phospho-peptide mapping studies and studies in cell lines (55). However, these tyrosine mutations still result in severe defects in downstream signaling pathways consistent with defective Vav1 or Itk activity. Similarly, mutation of tyrosines on Vav1 does not result in a loss of interaction with their proposed binding partners, although they do result in abrogation of Vav1-dependent signaling (53). While the continued interactions of these proteins seen by immunoprecipitation experiments likely may be due to tertiary interactions with other domains or other molecules, these studies suggest that SH2/phosphotyrosine interactions may play important regulatory roles for the activation of effector molecules. Indeed, structural studies have suggested that the interaction between the SH2 domain of Itk and a phosphotyrosine results in a conformational switch allowing kinase activity (56). Consistent with these data, it was recently shown in Jurkat T cell lines that an Itk/SLP-76 interaction is required for Itk kinase activity, although it has not yet been shown if it is specifically the SH2/phosphotyrosine interaction that mediates this kinase activity (57). Further studies are required to determine how the activities of molecules beyond Itk are affected by specific domain-domain interactions within the complex.
The proximal signaling complex results in the activation of PLCγ1-dependent pathways including Ca2+ and DAG-induced responses, cytoskeletal rearrangements, and integrin activation pathways. Ligation of costimulatory receptors such as CD28 augments these pathways. Below we discuss the mechanisms by which these pathways are activated and regulated.
PLCγ1 Activation and Signal Transduction
Following TCR ligation, PLCγ1 is found in the proximal signaling complex bound to SLP-76, Vav1, and LAT, where it is phosphorylated and activated by Itk. Activated PLCγ1 then hydrolyzes the membrane lipid PI(4,5)P2 (phosphatidylinositol 4,5 bisphosphate) producing the second messengers IP3 and DAG. These two messengers are essential for T cell function, and therefore the regulation of PLCγ1 activation has been the subject of intensive studies. Localization of PLCγ1 to the proximal signaling complex is dependent on LAT and the Gads-binding region of SLP-76 (52). Activation of PLCγ1 is dependent on Itk kinase activity that, in turn, is dependent on Vav1, lck, ZAP-70, LAT, and SLP-76 (50, 58, 59). Following TCR ligation, Itk is recruited to the membrane through PH domain interactions with PIP3, which has been locally generated by lck-induced PI3K activity (reviewed in 59). At the membrane, lck phosphorylates Itk, and the SH2 and SH3 domains of Itk interact with phosphorylated tyrosine 145 and the PRR of SLP-76, respectively (60–62). The role of Vav1 and ZAP-70 in Itk activation is not understood but may relate to their involvement in the phosphorylation of SLP-76 or PI3K activation (43, 49, 50). A second tec family kinase, Rlk, can also phosphorylate PLCγ1, resulting in a relatively mild defect in Itk-deficient mice and requiring the study of Rlk/Itk double deficient mice to better understand the role of tec kinases in T cell activation (reviewed in 59).
DAG-Mediated Signaling Pathways
TCR-induced production of DAG results in the activation of two major pathways involving Ras and PKCθ. Ras is a guanine nucleotide-binding protein and is required for the activation of the serine-threonine kinase Raf-1 that leads to the activation of the mitogen-associated protein kinases (MAPKs) extracellular signal-regulated kinase 1 (Erk1) and Erk2. Erk activation results in transcriptional activation of Elk1 and signal transducer and activator of transcription 3 (STAT3) and lck serine phosphorylation (reviewed in 63). Ras is also involved in activation of the activator protein-1 (AP-1) (c-Jun/c-Fos) transcription complex and upregulation of CD69 expression (64). Ras is only active in the GTP-bound state, and its activation is facilitated by GEFs and suppressed by GTPase activating proteins (GAPs). Two Ras GEFs are present in T cells, son of sevenless (SOS) and Ras guanyl nucleotide-releasing protein (RasGRP). RasGRP appears more dominant for early activation of Ras, as SOS cannot compensate for RasGRP deficiency (65–67). RasGRP is inducibly recruited to the membrane through a DAG-binding domain (68), where it is phosphorylated by PKCθ (69). SOS is constitutively bound to the adapter protein GRB2, and upon TCR stimulation, the GRB2 SH2 domain is recruited to and binds phosphorylated tyrosines on LAT, thereby bringing SOS into the proximal signaling complex where it can facilitate the localized activation of Ras (70). The significance of these two modes of Ras activation was unclear until recently, when it was shown that RasGRP-dependent RasGTP production catalyzes SOS activity, resulting in a positive feedback loop and robust TCR-induced Ras activation (71) (Figure 1c). This unified model explains the RasGRP dominance, as RasGRP would be required to initiate the production of RasGTP, which upon reaching threshold levels would catalyze SOS activity and further amplify the signal.
The second major signaling pathway regulated by DAG is mediated by PKCθ, a PKC family member that contains a lipid-binding domain specific for DAG, which is important for recruiting PKCθ to the plasma membrane following T cell activation. However, it has been proposed that phosphorylation by lck (reviewed in 72) may be required to induce a conformational change that enables binding to the lipid phosphatidyl serine (PS), which in turn enhances binding to DAG, resulting in PKCθ activation (73). Other proximal signaling molecules, including Vav1, PI3K, and 3-phosphoinositide-dependent kinase 1 (PDK1), also play a role in PKCθ localization, but details of their contributions are not completely defined (72).
One critical pathway that PKCθ regulates is NFκB activation. Since both the canonical and non-canonical activation of this pathway in T cells is described in detail in this volume of Annual Review of Immunology (see review by Karin and colleagues), we highlight only the major steps in the classical activation of NFκB downstream of the TCR. The NFκB family of transcription factors consists of five members. In resting cells, NFκB is found in the cytosol associated with inhibitor of NFκB (IκB) family members that keep NFκB from moving into the nucleus. Upon T cell activation, IκB is phosphorylated by the IκB kinase (IKK) complex, ubiquitinylated, and degraded, allowing NFκB to translocate into the nucleus, where it actives genes involved in the function, survival, and homeostasis of T cells (reviewed in 74). While the general pathway of NFκB activation has been known for some time, the specifics of how PKCθ activation leads to nuclear import of NFκB in T cells is still being elucidated.
Over the past several years, the identification and characterization of a lymphocyte-specific activation complex has provided some insight into this question. Following TCR stimulation, a trimolecular complex forms between CARMA1 [caspase recruitment domain (CARD) and membrane-associated guanylate kinase (MAGUK)-containing scaffold protein], the CARD-containing adapter protein Bcl10, and mucosa-associated lymphoid tissue lymphoma translocation gene 1 (MALT1) (72, 74). The assembly of this complex is regulated by PKCθ through its phosphorylation of CARMA1, which is required for CARMA1 oligomerization and association with Bcl10 (75, 76). MALT1 binds to Bcl10 and contributes to the degradation of the regulatory subunit of the IKK complex (IKKγ) by facilitating its polyubiquitination, possibly via activation of the E3 ubiquitin ligase tumor necrosis factor receptor-associated factor 6 (TRAF6) (77, 78). Degradation of this regulatory subunit allows for phosphorylation of IκB by the IKK catalytic subunits, subsequent IκB degradation, and release of NFκB, resulting in NFκB nuclear localization and gene activation. Recently, MALT1 was shown to enhance NFκB signaling through its ability to degrade the deubiquitinating enzyme A20, a negative regulator of NFκB activation (79, 80).
One additional component of the complex that was recently identified as a CARMA1-associating protein is ADAP (81). ADAP, originally defined as a SLP-76-binding partner, associates with the MAGUK domain of CARMA1 following T cell stimulation. It was proposed that this interaction might alter the conformation of CARMA1 and enhance its association with Bcl10 and MALT1 and/or stabilize the CARMA1/Bcl-10/MALT1 complex.
Ca2+-Mediated Signaling Pathways
Ca2+ions are universal second messengers in eukaryotic cells. An extensive review on Ca2+ signaling in T cells can be found in this volume of Annual Review of Immunology (see review by Rao and colleagues). The IP3 generated by TCR-stimulated PLCγ1 activity stimulates Ca2+-permeable ion channel receptors (IP3R) on the endoplasmic reticulum (ER) membrane, leading to the release of ER Ca2+ stores into the cytoplasm. Depletion of ER Ca2+ triggers a sustained influx of extracellular Ca2+ through the activation of plasma membrane Ca2+ release-activated Ca2+ (CRAC) channels in a process known as store-operated Ca2+ entry (SOCE) (reviewed in 82) (Figure 1d). For decades the CRAC channels had only been identified by their biophysical properties, and it has just been in the past few years that the pore-forming subunit of the channels was identified as the four transmembrane domain-containing molecule ORAI (83–85). Additionally, studies in the past few years have revealed the sensor for depleted ER Ca2+ stores and the activator of CRAC channels as stromal interaction molecule (STIM) (86, 87). STIM is a transmembrane protein with protein interaction domains both within the ER lumen and in the cytoplasm and paired EF hands located in the ER lumen, where one hand binds Ca2+ with low affinity (86, 87). Two STIM proteins, STIM1 and STIM2, are found in mammals, and recent work has shown that STIM1 is important for the initial robust phase of SOCE, whereas STIM2 is important for maintaining basal Ca2+ levels and sustaining the late phase of SOCE (88, 89). Following ER Ca2+ depletion, STIM1 molecules aggregate in clusters that preferentially localize to sites of ER plasma membrane apposition, where they co-localize with clusters of ORAI (90–92). Whether STIM1 and ORAI interact directly or indirectly remains controversial. While these two proteins are clearly necessary for TCR-induced SOCE, the biochemical mechanisms by which ER depletion is coupled to CRAC activation remains to be fully understood. While CRAC channels appear to be the dominant mode of Ca2+ entry in T cells, other Ca2+ channels exist; however, their relevance remains unclear (reviewed in 82).
TCR-induced increases in intracellular Ca2+ levels result in the activation of Ca2+ and calmodulin-dependent transcription factors, including myocyte-enhancing factor 2 (MEF2) and downstream regulatory element antagonist modulator (DREAM), as well as signaling proteins, including the phosphatase calcineurin and the Ca2+-calmodulin-dependent kinase (CaMK), that in turn activate a variety of transcription programs (reviewed in 93). Activated calcineurin dephosphorylates members of the nuclear factor of activated T cells (NFAT) family, leading to their translocation to the nucleus. In the nucleus, NFATs can form cooperative transcriptional complexes with a variety of transcription factors, thereby integrating signaling pathways resulting in differential transcriptional patterns. The most well studied interaction is NFAT/AP-1 that results in IL-2 production; however, the regulatory T cell lineage-specific transcription factor forkhead box protein 3 (FOXP3) also has been shown recently to cooperate with NFAT (94). Other pathways including NFκB and Jnk have been shown to be Ca2+ dependent; however, these effectors require additional signals, again emphasizing the crosstalk between Ca2+ and other T cell signaling pathways (95).
Actin and Cytoskeletal Responses
When a T cell is presented with cognate antigen by an APC, signals from the TCR initiate a program of actin cytoskeletal rearrangements that results in polarization and activation of the T cell (reviewed in 96). Actin reorganization is essential for T cell function, as actin polymerization inhibitors impede T cell/APC interactions (97) and abolish proximal TCR signals (98).
T cell/APC conjugation results in morphological changes, as the stimulated T cell rounds up and accumulates filamentous actin (F-actin) at the stimulatory interface. These changes are thought to be dependent on a TCR-induced increase in plasma membrane fluidity and a decrease in cellular motility. Cessation of motility is associated with the TCR and Ca2+-dependent phosphorylation and deactivation of the myosin motor protein MyH9; however, the signaling pathway linking the TCR to this event has not yet been defined fully (99). Plasma membrane fluidity is increased, in part, by the TCR- and Vav1-dependent transient dephosphorylation of ERM (ezrin, radixin, and moesin) proteins, resulting in the loss of their ability to link the plasma membrane to the actin cytoskeleton (100). Ca2+ signaling and integrin activation downstream of the TCR results in additional modifications of actin-associated proteins that may play roles in altering plasma membrane rigidity (96).
Accumulation of F-actin at the T cell/APC interface is the result of TCR-induced localized activation of multiple actin regulatory and polymerizing pathways, the best studied of which involves the actin-related proteins 2/3 (Arp2/3) complex, although Arp2/3-independent pathways also contribute to this process(101). Activation of Arp2/3 requires its interaction with nucleation-promoting factors (NPF) including Wiskott-Aldrich syndrome protein (WASp), WASp family verprolin homologous protein (Wave2), and hematopoietic cell lineage specific protein 1 (HS1). WASp is recruited to the site of TCR activation through its interaction with the SLP-76-associated adapter protein Nck, where it is activated via Vav1-dependent stimulation of the Rho-family GTPase Cdc42 (102). Like WASp, Wave2 activation is dependent on Vav1; however, in this case it is through Vav1-mediated stimulation of a second Rho-family GTPase, Rac1 (96). Given the proposed reliance of WASp and Wave2 on Vav1-mediated GTPase activation, it is interesting that actin-dependent processes that are defective in Vav1-deficient T cells can be rescued with the expression of a GEF-inactive Vav1 mutant, suggesting that other Rac and Cdc42 GEFs may be able to support TCR-induced actin changes (53). This result also suggests that other Vav-1 functions are important for TCR-induced actin changes. Consistent with this is the observation that through its protein interaction domains, Vav1 may contribute to Wave2 and WASp activation through the recruitment of Dynamin2, a GTPase known to be important for TCR-induced actin dynamics (103).
Activation of the T cell in response to an APC also results in the polarization of the T cell, whereby the microtubular organizing center (MTOC) moves towards the T cell/APC contact site (104). While polarization of the MTOC has long been observed as a hallmark of productive T cell/APC conjugation, the signaling mechanisms responsible for this movement remain undefined. Recent data suggest, however, that the adapter protein ADAP (a component of the SLP-76-nucleated complex) may play a role through its interaction with the microtubule motor protein dynein (105). Movement of the MTOC appears essential for the formation of the immunological synapse (IS). The IS is an organized structure that develops at the contact site between the T cell and the APC. It is composed of two concentric regions based on molecular composition: the TCR-rich central supramolecular activation cluster (cSMAC), surrounded by the integrin-rich peripheral SMAC (pSMAC) (106, 107). Although the IS was described approximately 10 years ago, its precise role in T cell activation remains unclear. Models have been proposed suggesting it is essential for both signal initiation and signal termination, while others suggest that the IS is irrelevant for signaling but essential instead for directed secretion of cytokines (reviewed in 108). It is likely that the IS is important for different aspects of each of these processes, likely depending on the precise conditions of cellular activation.
pMHC/TCR ligation also results in the formation of intracellular microclusters that contain the TCR complex and associated signaling molecules including LAT and SLP-76 (107, 109). These clusters initiate and sustain Ca2+ signals. The clusters persist for a short time, after which they converge towards the center of the T cell/APC interface at the center of the IS (107, 110, 111). Formation and translocation of the clusters is dependent on F-actin dynamics, and new clusters continue to form even after the mature IS is established (110–112). Integrins play a key role in sustaining microclusters, emphasizing the importance of integrin activation for T cell function (113).
Opposite the MTOC and IS another ordered structure forms, known as the distal pole complex (DPC). Although the role of the DPC is not known for certain, it is speculated that it is critical to sequester negative regulators away from the TCR activation complex (96). Additionally, the DPC may contribute to the polarization of key signaling molecules that may be required for distinguishing memory versus effector fate decisions in recently divided cells (114). Formation of this complex is dependent on F-actin and the rephosphorylation of ERM proteins that migrate to the distal pole linking signaling molecules to the cytoskeleton (115).
TCR signaling cascades and pathways downstream of actin reorganization are intertwined and difficult to tease apart, as many of the effector molecules involved have multiple enzymatic and adapter functions. WASp, Wave2, and Vav1 signals play roles in TCR-induced signaling that appear to be independent of their roles in actin responses (50, 116, 117). Therefore, loss of different actin regulators may result in complex TCR signaling defects (118). Future studies are required to fully understand the feedforward and feedback mechanisms that define the interdependence between cytoskeletal dynamics and T cell activation.
TCR Inside-Out Signaling to Integrins
Integrins are αβ heterodimeric receptors responsible for mediating cell/cell or cell/matrix adhesions. Key T cell integrins include leukocyte function-associated antigen-1 (LFA-1) and very late antigen-4 (VLA-4), which bind their respective ligands intercellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM) and fibronectin on other immune cells, endothelial cells, fibroblasts, and extracellular matrix proteins. Activation of integrins (increasing their affinity and avidity for ligand) is critically dependent upon biochemical events initiated by the TCR, a process designated “inside-out signaling” (119). Although the pathway from the TCR to integrin activation has not been completely elucidated, TCR-mediated activation of several key signaling molecules as well as TCR-induced actin/cytoskeletal changes have been implicated in this process.
A central regulator of inside-out signaling is the small GTPase Ras-proximity-1 (Rap1). Rap1 enhancement of T cell activation is thought to be through its critical role in mediating TCR-induced adhesion to ICAM-1. This conclusion is based on studies utilizing overexpression of dominant-negative and constitutively active forms of Rap1 (120, 121) and more recently by analysis of mice deficient in Rap1A, in which TCR-induced adhesion to ICAM-1 is markedly reduced (122). The importance of understanding the role of Rap1 is clear, as mutations in Rap1-mediated integrin activation have been linked to leukocyte adhesion-deficiency syndrome, a disease that can lead to severe bacterial infections (reviewed in 119).
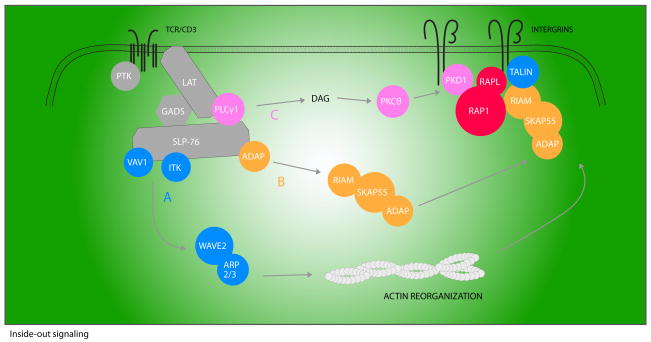
Many proximal signaling molecules that comprise the early TCR signalosome, including LAT, SLP-76, and PLCγ1, are required for integrin and Rap1 activation (reviewed in 119) (Figure 2). However, there are downstream effectors that have a more selective role in integrin activation, including the adapter protein ADAP. T cells from ADAP-deficient mice are defective in TCR-induced LFA-1 clustering and adhesion to ICAM (123). The association of ADAP with SLP-76 appears to be required for integrin activation, as overexpression of ADAP but not a mutant of ADAP that cannot bind to SLP-76 enhances integrin function following TCR ligation (124, 125). Although mice expressing the reciprocal mutation in the SH2 domain of SLP-76 have been generated and share several characteristics with ADAP-deficient mice, TCR-induced integrin activation in these mice has not yet been reported.
Figure 2. A model of integrin activation.
Upon TCR ligation, the LAT/SLP-76-nucleated signalosome assembles. This complex allows for the activation of three pathways necessary for inside-out activation of integrins. Vav1 and Itk contribute to the actin reorganization required for integrin mobility (pathway A). Mobilization of the ADAP/SKAP-55/RIAM complex is necessary for activated Rap1 plasma membrane localization (pathway B). An active Rap1/RIAM complex induces the association of talin with integrin β tails, perhaps resulting in altered integrin affinity. Thirdly, PLCγ1-mediated activation of DAG leads to PKD1 activation and association with Rap1 (pathway C). This interaction is required for Rap1 activation and contributes to Rap1 recruitment. Rap1 in turn recruits RAPL, and subsequent RAPL binding to αL subunits results in integrin clustering and affinity changes.
In addition to its inducible interaction with SLP-76, ADAP constitutively associates with Src kinase-associated phosphoprotein of 55 kDa (SKAP-55). The ADAP/SKAP55 complex is important for proper localization of activated Rap1 (124). How ADAP and SKAP-55 recruit Rap1 to the membrane was unclear until recently when it was shown that a third adapter, Rap1-GTP interacting adapter molecule (RIAM), which is also constitutively bound to SKAP-55, associates with activated Rap1 upon TCR ligation resulting in Rap1 movement to the membrane (126). How RIAM itself relocalizes to the plasma membrane following T cell stimulation is currently unknown, although it is hypothesized that recruitment may be through the inducible interaction of phosphoinositide PI(3,4)P2 (a product of T cell activation) and the pleckstrin homology (PH) domain of RIAM (127).
In addition to mobilization of the ADAP/SKAP-55/RIAM complex that is downstream of early TCR signals and likely SLP-76 dependent, Rap1-mediated integrin activation is also dependent upon other TCR-triggered signals, including those leading to activation of PKC. One PKC target important for Rap1 activation is the serine-threonine kinase protein kinase D 1 (PKD1) (also known as PKCμ). Following TCR stimulation, Rap1 associates with the PH domain of PKD1 (128). Interestingly, it is this interaction and not the kinase activity of PKD1 that is required for Rap1 membrane recruitment and activation. Since PKD1 also inducibly recruits the Rap1 GEF C3G (Crk SH3 domain GEF) to the membrane in a TCR-dependent fashion, it is tempting to speculate that this event contributes to the dependence of Rap1 on PKD1 for its activation (128).
Activated Rap1 can also associate with the effector regulator of cell adhesion and polarization enriched in lymphoid tissues (RAPL). This interaction is coincident with RAPL membrane localization and necessary for binding of RAPL to the αL-subunit of LFA-1. In cell line models, this association is important for LFA-1 clustering as well as affinity modulation (129).
In addition to regulating the activation of Rap1, signals from the TCR also regulate cytoskeletal attachments to integrins. One cytoskeletal binding protein important for integrin activation is talin. Recent studies in platelets have demonstrated that Rap1 activity enhances the association of talin with β-integrin subunits through association with RIAM (130). It was suggested that formation of a talin/RIAM/Rap1 complex enables talin to bind integrins, which may induce the high affinity ligand-binding state. Talin is not the only actin-binding protein implicated in TCR-induced integrin activation. Vinculin, WAVE2, and the Arp2/3 complex also play a role in this process, and defining the precise steps that link early TCR signals to activation of these molecules is an area of active investigation.
Costimulation
One central tenet of T cell activation is that signaling solely through the TCR results in a non-responsive state (anergy) in which T cells fail to respond and are then refractory to restimulation. Co-ligation of other cell surface receptors provides additional signals required for anergy avoidance and productive T cell activation. Although many cell surface receptors can enhance signaling through the TCR, CD28 does so more robustly than other costimulatory molecules.
Numerous studies have shown that CD28 promotes T cell proliferation, cytokine production (via gene transcription and mRNA stability), cell survival, and cellular metabolism (reviewed in 131). One key effector downstream of CD28 is PI3K. Following CD28 ligation by its ligands CD80 or CD86 on APCs, the p85 regulatory subunit of PI3K associates with a pYMNM motif located in the cytoplasmic tail of CD28 (132). This regulatory subunit recruits the p110 catalytic subunit of PI3K, which converts PIP2 to phosphatidylinositol (3,4,5) trisphosphate (PIP3) at the membrane. Localized PIP3 generation serves as a docking site for the PH domains of PDK1 (3-phosphoinositide-dependent protein kinase 1) and its target Akt.
Akt phosphorylates multiple proteins enabling it to affect numerous cellular responses. Activation of Akt enhances the nuclear translocation of NFκB, which has positive effects on the expression of pro-survival genes including Bcl-xl. This function, coupled to the ability of Akt to inhibit transcription factors that promote cell cycle arrest, results in Akt-driven cell survival and proliferation (131). Akt also affects optimal transcription of NFAT regulated genes, such as IL-2. One well-known target of Akt is GSK-3 (glycogen-synthase kinase 3), a serine-threonine kinase that promotes nuclear export of NFAT (133). Thus, inactivation of GSK-3 by Akt might be one pathway responsible for prolonged NFAT nuclear localization and thus IL-2 transcription following CD28 costimulation. Recently, a GSK-3-independent mechanism by which Akt may regulate NFAT activity was suggested. This model posits that phosphorylated NFAT is bound by the scaffolding protein Homer, thus inhibiting access of calcineurin to NFAT (134). CD28 ligation was proposed to induce Akt-mediated phosphorylation of Homer, resulting in its dissociation from pNFAT. Unbound pNFAT would then be susceptible to calcineurin phosphatase activity and nuclear entry. Whether this pathway is Akt dependent remains to be rigorously tested. Lastly, TCR/CD28 co-ligation increases the cell surface expression of the insulin transporter Glut1, leading to increase glucose uptake and glycolysis (135, 136), also reported to be mediated by Akt. Together, these data provide a framework for how Akt mediates T cell growth and survival downstream of CD28.
The CD28-mediated generation of PIP3 also serves as a docking site for the PH domain of Itk. Although Itk inducibily associates with the LAT/SLP-76/Gads/PLCγ1 complex that forms following TCR ligation, its localization and activation is also dependent upon PI3K generated PIP3 (59). Itk can also associate directly with CD28 via the CD28 proximal PxxP motif (59). It has been proposed that this interaction keeps Itk in close proximity to lck (which binds to the distal PxxP motif of CD28), allowing for lck-mediated phosphorylation and activation of Itk (137) and enhanced Ca2+ flux, another characteristic of CD28-mediated signaling. Although the proline motifs in the tail of CD28 are required for CD28-mediated proliferation and IL-2 production, these motifs are dispensable for Bcl-xl upregulation (138). This function appears to be more reliant upon the proximal YMNM p85 binding site (138). Thus, CD28 can differentially regulate proliferation and survival in activated T cells.
Another more recently described function of CD28 is induction of arginine methylation. Following CD28 ligation, protein arginine methyltransferase activity increases, and arginine methylation of multiple proteins, including Vav1, is induced. Vav1 arginine methylation appears to occur in the nucleus and correlates with IL-2 production (139). While the precise biologic significance of this posttranslational modification is unknown, this pathway may provide yet another mechanism by which CD28 regulates TCR signaling.
Many of the pathways described above are activated by TCR ligation alone; however, the magnitude of the response is considerably augmented with CD28 co-ligation. This has led to speculation that CD28 engagement results primarily in a quantitative rather than qualitative change in T cell activation parameters (131). While this appears to be true for a number of signaling pathways, sufficient data have accumulated to support the notion that CD28 binding also results in outcomes that are qualitatively distinct from those seen with TCR stimulation alone.
CD28-deficient mice exhibit dampened immune responses to a variety of infectious agents (reviewed in 131). While these studies demonstrate the importance of costimulation by CD28, not all immune responses are severely impacted by its loss (140). Such observations indicate that molecules other than CD28 can provide costimulation for T cells. Indeed, multiple surface receptors have been described as having costimulatory functions. Included among these are CD2, CD5, CD30, 4-1BB, OX40, inducible costimulator (ICOS), and LFA-1. For this review, we focus on the CD28-related protein ICOS and two members of the tumor necrosis factor receptor (TNFR) family to provide examples of how costimulatory molecules can link to downstream effectors, either directly or through adapter proteins.
Unlike CD28, which is expressed at constant levels on both resting and activated T cells, ICOS is inducibly expressed on activated T cells (141). ICOS deficiency results in impaired immune responses, similar to yet not as severe as those observed in CD28 knock-out models, suggesting that these two molecules may function in similar pathways (142). Indeed, ICOS shares several structural features with CD28 including a YMXM motif in its cytoplasmic tail that associates with p85 (142). This site is presumed to be responsible for PI3K-driven Atk and/or Itk activation observed downstream of CD3/ICOS stimulation, which likely contributes to the similarities seen in CD3/CD28 and CD3/ICOS-stimulated cells (143). ICOS does not induce IL-2 gene transcription as CD28 does. Failure to induce IL-2 has been attributed, at least in part, to the inability of the YxxM motif of ICOS to associate with Grb2, an association that is present via this motif in CD28 (144). Therefore, although ICOS activates genes similar to those induced by CD28, there are differences in the degree to which particular genes are expressed. These differences have in vivo relevance, as mice doubly deficient in CD28 and ICOS are severely defective in generating immune responses (145).
Outside of the CD28 family of costimulatory molecules, the OX40 (CD134) and 4-1BB (CD137) members of the TNFR family provide costimulation upon engagement with their ligands OX40L and 4-1BBL. Like CD28, OX40 or 4-1BB ligation induces activation of PI3K/Akt, NFκB, JNK, and p38 MAPK (146). However, unlike CD28 and ICOS, OX40 and 4-1BB do not directly associate with protein kinases but rather link to downstream signaling through the TRAF (TNFR-associated factor) family of adapter proteins. The proteins involved in linking TRAF signaling to NFκB and the JNK/p38 pathways have not been completely elucidated in primary T cells. However, studies in other systems implicate TRAFs themselves in the direct recruitment of the IKK complex as well as serine/threonine kinases that initiate signaling to JNK and p38 (reviewed in 147).
Many of the same genes are regulated downstream of CD28 and TNFR family members. One reason for such overlap may be due to the timing of receptor expression. CD28 is expressed early and is critical for induction of an immune response. It promotes expression of several other costimulatory molecules including ICOS, OX40, and 4-1BB. Once expressed, these receptors prolong or sustain an immune response, and in the case of OX40 and 4-1BB, are important for memory T cell formation (146). The use of alternative means by which to active these similar pathways, e.g. direct binding to kinases versus use of adapter proteins, may also allow for differential negative regulation of these pathways. In fact, some TRAF proteins negatively regulate NFκB; thus, association of TNFRs with different TRAF family members can modulate the immune response (147). Lastly, a role for many costimulatory molecules, including ICOS and 4-1BB, in the development and/or function of regulatory T cells is becoming increasingly apparent (148). This layer of complexity will have to be taken into account when deciphering the roles of costimulatory molecules in vivo.
Negative Regulation of TCR Signaling
As outlined above, signaling through the TCR triggers an array of signals that activate multiple effector pathways. Activation of these pathways is regulated to ensure that T cells respond to appropriate ligands and for the proper duration. As with positive regulation of T cell signaling, negative regulation is mediated through both TCR-generated signals and those emanating from other cell surface receptors (Table 2).
Table 2.
Sampling of inhibitors of TCR signaling
| Molecule | Interactions and/or function | Deficiency phenotype |
|---|---|---|
| Csk(149, 150) (Kinase) |
|
|
| HPK1 (156, 157) (Kinase) |
|
|
| SHP1 (151, 152) (Phosphatase) |
|
|
| Sts-1 (158, 159, 161) (Phosphatase) |
|
|
| DoK-1, Dok-2 (154, 155) (adapters) |
|
|
| c-Cbl, Cbl-b (162–164) (E3 ligase) |
|
|
| CTLA-4 (166, 169) (inhibitory receptor) |
|
|
| PD-1 (167, 168) (inhibitory receptor) |
|
|
Even the most proximal TCR signaling events are actively regulated. For example, multiple proteins contribute to the regulation of lck activity. C-terminal src kinase (Csk) is responsible for phosphorylating lck on its inhibitory tyrosine residue (Y505) and maintaining lck in an inactive state (149, reviewed in 150). Countering this is the phosphatase CD45 that dephosphorylates the inhibitory site allowing for lck autophosphorylation and activation. Interestingly, CD45 can also limit lck activity by dephosphorylating its active site (150). Whether CD45 negatively or positively impacts TCR signaling is likely to be controlled by its proximity to TCR-stimulated effector molecules during TCR engagement and whether CD45 itself is in an enzymatically favorable conformation.
An additional layer of lck regulation initiated by TCR signals has been proposed. As a means to explain how the TCR can distinguish between strong and weak ligands, it was shown that weak or antagonistic TCR ligation results in rapid lck-mediated phosphorylation of SHP1 (SH2 domain-containing protein-tyrosine phosphatase) (151). SHP1 then dephosphorylates the active site of lck, resulting in cessation of the TCR signal. Conversely, in the presence of strong or agonistic TCR ligation, Erk is rapidly activated and phosphorylates lck on Ser59. This activity is thought to prevent SHP1 binding, thus keeping lck active to sustain TCR signals and further amplify Erk activity. The extent to which this regulatory loop operates in vivo in the context of agonist stimulation awaits the generation and analysis of mice harboring a mutation at the indicated lck serine residue.
The importance of SHP1 function is evident, as mice deficient in SHP1 develop severe autoimmunity (152). SHP1 can be recruited quickly to the TCR complex via its association with lck. However, SH2 domain-containing phosphatases are typically recruited to phosphorylated ITIMs (immunoreceptor tyrosine-based inhibitory motifs) present in the cytoplasmic tails of cell surface receptors. Recently, the ITIM-bearing inhibitory receptor carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) was identified as a potential candidate for SHP-1 recruitment in human T cells (153). CEACAM1 upregulation occurs hours after stimulation; thus, it is likely that SHP1 regulates initial TCR signaling events as well as late signaling termination events. Given the impact SHP1 activity has on immune regulation, it will be important to understand exactly how SHP1 is recruited into the TCR-ligated signaling complex.
Adapter proteins also play a critical role in negatively regulating TCR signals. The downstream of kinase (Dok) adapter proteins, Dok-1 and -2, are expressed in T cells. Coordinated deletion or knock-down of these proteins results in increased TCR cytokine production, proliferation, prolonged phosphorylation of ZAP-70, LAT, SLP-76, and Akt, and the development of a lupus-like renal disease with high anti-double-stranded DNA antibody titers (154, 155). Dok can associate with several negative regulators including SHIP-1, Csk, and RasGAP; however, how these associations mediate the negative regulatory role of Dok proteins remains to fully elucidated. Recent structure/function studies in T cell lines showed that the phosphotyrosine binding domain (PTB) of Dok-1 and -2 can bind the ITAM motif in CD3ζ, making it tempting to speculate that Dok may compete with ZAP-70 for ITAM binding (154). Whether this interaction is relevant in vivo remains to be shown. Dok proteins can also be recruited inducibly to a LAT, Grb2, and SHIP-1-containing molecular complex (155). This finding is intriguing and begs the question as to how LAT participates in both positive and negative signaling pathways. Determining the domains necessary for the assembly of this negative regulatory complex and whether it is separate from or a part of the stimulatory LAT-containing complex may provide insight into how early phosphorylation events are regulated.
It has been suggested that SLP-76 can also be a target of negative regulation. The serine/threonine kinase HPK1 inducibly binds to the SH2 domain of SLP-76. This kinase has been reported to have both positive and negative effects on TCR signal transduction; however, its role as a prominent negative regulator was confirmed recently by the description of HPK1-deficient mice. HPK1-deficient T cells exhibit enhanced phosphorylation of several early signaling molecules, and HPK1-deficient mice are more susceptible to experimental autoimmune encephalomyelitis (EAE) than wildtype mice (156). As a possible explanation for these phenotypes, two groups have shown that HPK1 can phosphorylate a serine residue in SLP-76 that mediates recruitment of 14-3-3 family members (156, 157). 14-3-3 proteins have been implicated in the regulation of several signaling pathways. Thus, although the precise mechanism by which HPK1 disrupts TCR signal transduction remains unclear, identification of a SLP-76/14-3-3 interaction may indicate that this mechanism involves a conserved method of signal transduction regulation.
The role of a novel family of proteins in regulating TCR signaling has become appreciated more recently. The suppressor of T cell receptor signaling (Sts) family of proteins contains two members, Sts-1 (TULA2) and -2 (TULA). Combined deficiency in these proteins leads to hyperproliferative T cells and an increased susceptibility to autoimmunity (158). When the phenotype of Sts-1 and -2 knockout mice was described, the mechanism of negative regulation was unclear. Although still not understood fully, recent studies indicate that the C-terminal phosphoglycerate mutase domain of Sts-1 acts as a phosphatase with specificity for Syk and, to a lesser degree, Src family members, thus providing a model for how Sts-1 may negatively regulate TCR signal transduction (159). Interestingly, Sts-2 has very little phosphatase activity and, in cell line models, can enhance ZAP-70 activation (160). Recent studies demonstrating that Sts-2 can bind to and induce the degradation of c-Cbl (a negative regulator of TCR signaling; see below) have provided a mechanism by which Sts-2 may act as a positive regulator of T cell activation (161). Whether net ZAP-70 activity is ultimately increased or decreased in response to an in vivo challenge likely depends on the relative expression of these two family members.
Cbl proteins play an important role as negative regulators of T cell signaling. Two family members, c-Cbl and Cbl-b, are immune modulators, as deletion of each gene in vivo leads to hypercellularity and, in the case of Cbl-b, spontaneous mulitorgan infiltration (162, 163). Both c-Cbl and Cbl-b facilitate the ubiquitination of proteins, targeting them for degradation. They also mediate the downregulation of the TCR following stimulation with antigenic peptides and have been recently shown to play a role in the dissipation of the early signaling complex (163–165). Thus, Cbl proteins, by way of their ability to regulate protein degradation, provide an example of yet another means by which TCR signaling is terminated.
Similar to the necessity of signals provided by the TCR to be complemented by costimulatory molecules, TCR-generated regulatory signals are also aided by co-receptor signals. Cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) are two examples of such receptors that limit the expansion and activation of TCR-triggered T cells. These molecules are found on activated T cells with peak expression 24–48 hours after stimulation. Genetic studies have documented the importance of both for maintaining self-tolerance. By 5–6 days after birth, CTLA-4-deficient mice exhibit activated peripheral T cells, splenomegaly, and lymphocytic infiltrates into non-lymphoid organs (166). Although less striking, PD-1-deficient mice also develop autoimmune features including the development of a lupus-like disease by 6 months of age (167). Like costimulatory receptors, inhibitory receptors utilize similar motifs and molecular pathways to those used by the TCR when propagating a negative signal; they may even provide docking sites for these shared molecules (168, 169). Another mode by which inhibitory receptors have been proposed to function is to utilize the same ligand and/or signaling molecules as costimulatory receptors, thus setting up the potential for competition or sequestration of ligands or key substrates. This means is best illustrated by CTLA-4 and CD28, which share the ligands CD80 and CD86 and counter each other in the regulation of cell cycle proteins, cytokine expression, and Cbl-b expression.
Conclusions
As in the first 10 years after identification of the TCR, the past 15 years have seen a dramatic expansion in our understanding of the biochemical pathways triggered downstream of the TCR. We now appreciate more fully how PTKs couple to effectors and second messengers via the utilization of adapter proteins, how Ras and NFκB pathways are activated, how Ca2+ ions are sensed, that TCR signaling can be required for the activation of other cell surface receptors, and that the actin cytoskeleton does more than just dictate cell shape. Studies investigating signal transduction by costimulatory molecules have highlighted the importance of both signal amplification and the induction of additional signaling pathways, and discoveries of spontaneous or induced models of autoimmunity have brought negative regulation of these signaling pathways to the forefront. However, as often noted in this review, links between many signaling molecules and cellular outcomes remain poorly defined. Future studies will be required to fill gaps in our knowledge and likely will reveal new and unexpected interactions between currently known and unknown molecules.
This review primarily focused on the signal transduction pathways in mature T cells. Over the next 15 years, it will be important to apply current and new knowledge to other T cell lineages, including T regulatory cells, natural killer T cells, and memory T cells, to establish whether these paradigms are universal. In the coming years, the population-based analysis that established the field of signal transduction will be driven towards single cell analysis. We can anticipate this technological shift will allow for better analysis of human T cells and the application of basic science to human disease.
Sidebar
Arguing by Analogy: Comparing and Contrasting Signaling Pathways by Multiple Receptors and Lineages
Key insights important for understanding T cell activation have come from studies in non-immune mammalian cells and cells of lower organisms, including the observation that PTKs could link to the phosphatidylinositiol pathway and the paradigm describing adapter proteins as integrators of signal transduction. T cell biologists have also provided unique insights to their non-immunologist colleagues. Examples include a mechanism for recruitment of non-receptor PTKs to enzymatically inactive surface receptors and the notion that protein tyrosine phosphatases may be positive as well as negative regulators of PTK pathways.
Within the immune system, biologists studying TCR signaling have been the donors and recipients of information that has been useful for investigators examining the signaling pathways of other cell surface receptors (e.g. integrins) and other hematopoietic lineages. It is clear that although basic paradigms may be similar, each cell type and receptor utilizes unique ways to regulate signal transduction cascades. Additionally, recent studies have demonstrated that pathways thought to be distinct (e.g. integrins and immunoreceptors) instead intersect at multiple levels. Future insights into how diverse signaling pathways are integrated to result in the appropriate biologic response in will undoubtedly continue to benefit from comparing and contrasting activation events downstream of multiple receptors in different immune and non-immune cell lineages.
Acknowledgments
We thank Christopher Garvin for figure design, Dr. Art Weiss for helpful comments and Justina Stadanlick for editorial assistance.
Mini glossary
- Signal transduction
Biochemical events linking surface receptor engagement to cellular responses
- ITAM
Immunoreceptor tyrosine-based activation motif, a short peptide sequence in the cytoplasmic tails of key surface receptors on hematopoietic cells. This motif is characterized by tyrosine residues that are phosphorylated by src family PTKs enabling the ITAM to recruit activated syk family kinases
- Adapter protein, Cellular protein that functions to bridge molecular interactions via characteristic domains able to mediate protein
protein or protein:lipid interactions
- Inside-out signaling
Signals initiated by engagement of immunoreceptors that lead to conformational changes and clustering of integrins thereby increasing the affinity and avidity of the integrins for their ligands
- Costimulation
Signals delivered to T cells by cell surface receptors other than the TCR itself that either potentiate or interfere with T cell activation
Important acronyms
- TCR
T cell antigen receptor
- ITAM
immunoreceptor tyrosine-based activation motif
- PKC
protein kinase C
- PLC
phospholipase C
- PTK
protein tyrosine kinase
- PI3K
phosphoinositide 3-kinase
- pMHC
peptide major histocompatibility complex (MHC) complex
- NFAT
nuclear factor of activated T cells
- cSMAC
central supramolecular activation cluster
- pSMAC
peripheral supramolecular activation cluster
LITERATURE CITED
- 1.Haskins K, Kappler J, Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. Annu Rev Immunol. 1984;2:51–66. doi: 10.1146/annurev.iy.02.040184.000411. [DOI] [PubMed] [Google Scholar]
- 2.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–74. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 3.Yanagi Y, Yoshikai Y, Leggett K, Clark SP, Aleksander I, Mak TW. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984;308:145–9. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- 4.Hedrick SM, Cohen DI, Nielsen EA, Davis MM. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984;308:149–53. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- 5.Malissen M, Minard K, Mjolsness S, Kronenberg M, Goverman J, Hunkapiller T, Prystowsky MB, Yoshikai Y, Fitch F, Mak TW, et al. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984;37:1101–10. doi: 10.1016/0092-8674(84)90444-6. [DOI] [PubMed] [Google Scholar]
- 6.Allison JP, McIntyre BW, Bloch D. Tumor-specific antigen of murine T-lymphoma defined with monoclonal antibody. J Immunol. 1982;129:2293–300. [PubMed] [Google Scholar]
- 7.Haskins K, Kubo R, White J, Pigeon M, Kappler J, Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med. 1983;157:1149–69. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meuer SC, Fitzgerald KA, Hussey RE, Hodgdon JC, Schlossman SF, Reinherz EL. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983;157:705–19. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borst J, Coligan JE, Oettgen H, Pessano S, Malin R, Terhorst C. The delta- and epsilon-chains of the human T3/T-cell receptor complex are distinct polypeptides. Nature. 1984;312:455–8. doi: 10.1038/312455a0. [DOI] [PubMed] [Google Scholar]
- 10.Dembic Z, Haas W, Weiss S, McCubrey J, Kiefer H, von Boehmer H, Steinmetz M. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986;320:232–8. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- 11.Saito T, Germain RN. Predictable acquisition of a new MHC recognition specificity following expression of a transfected T-cell receptor beta-chain gene. Nature. 1987;329:256–9. doi: 10.1038/329256a0. [DOI] [PubMed] [Google Scholar]
- 12.Weiss A, Stobo JD. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984;160:1284–99. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004;4:301–8. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 14.Weiss A, Imboden JB. Cell surface molecules and early events involved in human T lymphocyte activation. Adv Immunol. 1987;41:1–38. doi: 10.1016/s0065-2776(08)60029-2. [DOI] [PubMed] [Google Scholar]
- 15.Imboden JB, Stobo JD. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985;161:446–56. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samelson LE, Patel MD, Weissman AM, Harford JB, Klausner RD. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986;46:1083–90. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- 17.Samelson LE, Phillips AF, Luong ET, Klausner RD. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc Natl Acad Sci USA. 1990;87:4358–62. doi: 10.1073/pnas.87.11.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–8. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 19.Barber EK, Dasgupta JD, Schlossman SF, Trevillyan JM, Rudd CE. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci U S A. 1989;86:3277–81. doi: 10.1073/pnas.86.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.June CH, Fletcher MC, Ledbetter JA, Schieven GL, Siegel JN, Phillips AF, Samelson LE. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1990;87:7722–6. doi: 10.1073/pnas.87.19.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–4. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- 22.Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 23.Romeo C, Amiot M, Seed B. Sequence requirements for induction of cytolysis by the T cell antigen/Fc receptor zeta chain. Cell. 1992;68:889–97. doi: 10.1016/0092-8674(92)90032-8. [DOI] [PubMed] [Google Scholar]
- 24.Wegener AM, Letourneur F, Hoeveler A, Brocker T, Luton F, Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992;68:83–95. doi: 10.1016/0092-8674(92)90208-t. Three papers describing chimeric proteins demonstrating the signaling capability of CD3ζ, supporting the notion of CD3 as the signaling component of the TCR complex. [DOI] [PubMed] [Google Scholar]
- 25.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–62. doi: 10.1016/0092-8674(92)90598-7. Describes the cloning and initial characterization of ZAP-70, the syk family PTK essential for coupling the TCR to its downstream signaling machinery. [DOI] [PubMed] [Google Scholar]
- 26.Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–9. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 27.Aivazian D, Stern LJ. Phosphorylation of T cell receptor zeta is regulated by a lipid dependent folding transition. Nat Struct Biol. 2000;7:1023–6. doi: 10.1038/80930. [DOI] [PubMed] [Google Scholar]
- 28.Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–12. doi: 10.1016/s0092-8674(02)00799-7. The first ITAM-independent function for a CD3 molecule is identified in the PRR of CD3ε as a binding site for Nck. [DOI] [PubMed] [Google Scholar]
- 29.Mingueneau M, Sansoni A, Gregoire C, Roncagalli R, Aguado E, Weiss A, Malissen M, Malissen B. The proline-rich sequence of CD3epsilon controls T cell antigen receptor expression on and signaling potency in preselection CD4+CD8+ thymocytes. Nat Immunol. 2008;9:522–32. doi: 10.1038/ni.1608. [DOI] [PubMed] [Google Scholar]
- 30.Szymczak AL, Workman CJ, Gil D, Dilioglou S, Vignali KM, Palmer E, Vignali DA. The CD3epsilon proline-rich sequence, and its interaction with Nck, is not required for T cell development and function. J Immunol. 2005;175:270–5. doi: 10.4049/jimmunol.175.1.270. [DOI] [PubMed] [Google Scholar]
- 31.Gil D, Schrum AG, Daniels MA, Palmer E. A role for CD8 in the developmental tuning of antigen recognition and CD3 conformational change. J Immunol. 2008;180:3900–9. doi: 10.4049/jimmunol.180.6.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tailor P, Tsai S, Shameli A, Serra P, Wang J, Robbins S, Nagata M, Szymczak-Workman AL, Vignali DA, Santamaria P. The proline-rich sequence of CD3epsilon as an amplifier of low-avidity TCR signaling. J Immunol. 2008;181:243–55. doi: 10.4049/jimmunol.181.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi K, Yang H, Ng E, Park SY, Sun ZY, Reinherz EL, Wagner G. Structural and functional evidence that Nck interaction with CD3epsilon regulates T-cell receptor activity. J Mol Biol. 2008;380:704–16. doi: 10.1016/j.jmb.2008.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–66. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 35.Sun ZJ, Kim KS, Wagner G, Reinherz EL. Mechanisms contributing to T cell receptor signaling and assembly revealed by the solution structure of an ectodomain fragment of the CD3 epsilon gamma heterodimer. Cell. 2001;105:913–23. doi: 10.1016/s0092-8674(01)00395-6. [DOI] [PubMed] [Google Scholar]
- 36.Ma Z, Janmey PA, Finkel TH. The receptor deformation model of TCR triggering. Faseb J. 2008;22:1002–8. doi: 10.1096/fj.07-9331hyp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minguet S, Schamel WW. A permissive geometry model for TCR-CD3 activation. Trends Biochem Sci. 2008;33:51–7. doi: 10.1016/j.tibs.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Alarcon B, Swamy M, van Santen HM, Schamel WW. T-cell antigen-receptor stoichiometry: pre-clustering for sensitivity. EMBO Rep. 2006;7:490–5. doi: 10.1038/sj.embor.7400682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nat Immunol. 2006;7:803–9. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- 40.Varma R. TCR triggering by the pMHC complex: valency, affinity, and dynamics. Sci Signal. 2008;1:pe21. doi: 10.1126/stke.119pe21. [DOI] [PubMed] [Google Scholar]
- 41.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–43. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 43.Bubeck Wardenburg J, Fu C, Jackman JK, Flotow H, Wilkinson SE, Williams DH, Johnson R, Kong G, Chan AC, Findell PR. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol Chem. 1996;271:19641–4. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 44.Koretzky GA, Abtahian F, Silverman MA. SLP76 and SLP65: complex regulation of signalling in lymphocytes and beyond. Nat Rev Immunol. 2006;6:67–78. doi: 10.1038/nri1750. [DOI] [PubMed] [Google Scholar]
- 45.Sommers CL, Samelson LE, Love PE. LAT: a T lymphocyte adapter protein that couples the antigen receptor to downstream signaling pathways. Bioessays. 2004;26:61–7. doi: 10.1002/bies.10384. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, Long EO, Love PE, Samelson LE. Essential role of LAT in T cell development. Immunity. 1999;10:323–32. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 47.Liu SK, Fang N, Koretzky GA, McGlade CJ. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr Biol. 1999;9:67–75. doi: 10.1016/s0960-9822(99)80017-7. LAT−/− mice reveal a complete block in T cell development that, along with studies describing SLP-76−/− mice, exemplifies in vivo the essential role of adapter proteins for signal transduction. [DOI] [PubMed] [Google Scholar]
- 48.Dombroski D, Houghtling RA, Labno CM, Precht P, Takesono A, Caplen NJ, Billadeau DD, Wange RL, Burkhardt JK, Schwartzberg PL. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J Immunol. 2005;174:1385–92. doi: 10.4049/jimmunol.174.3.1385. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds LF, de Bettignies C, Norton T, Beeser A, Chernoff J, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos, and RasGRP1. J Biol Chem. 2004;279:18239–46. doi: 10.1074/jbc.M400257200. [DOI] [PubMed] [Google Scholar]
- 50.Reynolds LF, Smyth LA, Norton T, Freshney N, Downward J, Kioussis D, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and -independent pathways. J Exp Med. 2002;195:1103–14. doi: 10.1084/jem.20011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi Q, August A. Keeping the (kinase) party going: SLP-76 and ITK dance to the beat. Sci STKE. 2007;2007:pe39. doi: 10.1126/stke.3962007pe39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beach D, Gonen R, Bogin Y, Reischl IG, Yablonski D. Dual role of SLP-76 in mediating T cell receptor-induced activation of phospholipase C-gamma1. J Biol Chem. 2007;282:2937–46. doi: 10.1074/jbc.M606697200. [DOI] [PubMed] [Google Scholar]
- 53.Miletic AV, Sakata-Sogawa K, Hiroshima M, Hamann MJ, Gomez TS, et al. Vav1 acidic region tyrosine 174 is required for the formation of T cell receptor-induced microclusters and is essential in T cell development and activation. J Biol Chem. 2006;281:38257–65. doi: 10.1074/jbc.M608913200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sommers CL, Lee J, Steiner KL, Gurson JM, Depersis CL, et al. Mutation of the phospholipase C-gamma1-binding site of LAT affects both positive and negative thymocyte selection. J Exp Med. 2005;201:1125–34. doi: 10.1084/jem.20041869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jordan MS, Smith JE, Burns JC, Austin JE, Nichols KE, et al. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity. 2008;28:359–69. doi: 10.1016/j.immuni.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pletneva EV, Sundd M, Fulton DB, Andreotti AH. Molecular details of Itk activation by prolyl isomerization and phospholigand binding: the NMR structure of the Itk SH2 domain bound to a phosphopeptide. J Mol Biol. 2006;357:550–61. doi: 10.1016/j.jmb.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 57.Bogin Y, Ainey C, Beach D, Yablonski D. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc Natl Acad Sci U S A. 2007;104:6638–43. doi: 10.1073/pnas.0609771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu KQ, Bunnell SC, Gurniak CB, Berg LJ. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med. 1998;187:1721–7. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. Tec family kinases in T lymphocyte development and function. Annu Rev Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 60.Shan X, Wange RL. Itk/Emt/Tsk activation in response to CD3 cross-linking in Jurkat T cells requires ZAP-70 and Lat and is independent of membrane recruitment. J Biol Chem. 1999;274:29323–30. doi: 10.1074/jbc.274.41.29323. [DOI] [PubMed] [Google Scholar]
- 61.Bunnell SC, Diehn M, Yaffe MB, Findell PR, Cantley LC, Berg LJ. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem. 2000;275:2219–30. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 62.Su YW, Zhang Y, Schweikert J, Koretzky GA, Reth M, Wienands J. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur J Immunol. 1999;29:3702–11. doi: 10.1002/(SICI)1521-4141(199911)29:11<3702::AID-IMMU3702>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 63.Genot E, Cantrell DA. Ras regulation and function in lymphocytes. Curr Opin Immunol. 2000;12:289–94. doi: 10.1016/s0952-7915(00)00089-3. [DOI] [PubMed] [Google Scholar]
- 64.D’Ambrosio D, Cantrell DA, Frati L, Santoni A, Testi R. Involvement of p21ras activation in T cell CD69 expression. Eur J Immunol. 1994;24:616–20. doi: 10.1002/eji.1830240319. [DOI] [PubMed] [Google Scholar]
- 65.Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, Barry M, Bleakley RC, Ostergaard HL, Stone JC. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95:3199–203. [PubMed] [Google Scholar]
- 66.Egan SE, Giddings BW, Brooks MW, Buday L, Sizeland AM, Weinberg RA. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993;363:45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- 67.Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–21. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 68.Ebinu JO, Bottorff DA, Chan EY, Stang SL, Dunn RJ, Stone JC. RasGRP, a Ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–6. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 69.Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol Cell Biol. 2005;25:4426–41. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–26. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 71.Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol Cell Biol. 2007;27:2732–45. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hayashi K, Altman A. Protein kinase C theta (PKCtheta): a key player in T cell life and death. Pharmacol Res. 2007;55:537–44. doi: 10.1016/j.phrs.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Melowic HR, Stahelin RV, Blatner NR, Tian W, Hayashi K, et al. Mechanism of diacylglycerol-induced membrane targeting and activation of protein kinase Ctheta. J Biol Chem. 2007;282:21467–76. doi: 10.1074/jbc.M700119200. [DOI] [PubMed] [Google Scholar]
- 74.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor kappa B. Immunity. 2006;25:701–15. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Matsumoto R, Wang D, Blonska M, Li H, Kobayashi M, et al. Phosphorylation of CARMA1 plays a critical role in T Cell receptor-mediated NF-kappaB activation. Immunity. 2005;23:575–85. doi: 10.1016/j.immuni.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 76.Sommer K, Guo B, Pomerantz JL, Bandaranayake AD, Moreno-Garcia ME, et al. Phosphorylation of the CARMA1 linker controls NF-kappaB activation. Immunity. 2005;23:561–74. doi: 10.1016/j.immuni.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 77.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 78.Zhou H, Wertz I, O’Rourke K, Ultsch M, Seshagiri S, et al. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature. 2004;427:167–71. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- 79.Coornaert B, Baens M, Heyninck K, Bekaert T, Haegman M, et al. T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-kappaB inhibitor A20. Nat Immunol. 2008;9:263–71. doi: 10.1038/ni1561. [DOI] [PubMed] [Google Scholar]
- 80.Rebeaud F, Hailfinger S, Posevitz-Fejfar A, Tapernoux M, Moser R, et al. The proteolytic activity of the paracaspase MALT1 is key in T cell activation. Nat Immunol. 2008;9:272–81. doi: 10.1038/ni1568. [DOI] [PubMed] [Google Scholar]
- 81.Medeiros RB, Burbach BJ, Mueller KL, Srivastava R, Moon JJ, et al. Regulation of NF-kappaB activation in T cells via association of the adapter proteins ADAP and CARMA1. Science. 2007;316:754–8. doi: 10.1126/science.1137895. [DOI] [PubMed] [Google Scholar]
- 82.Oh-Hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20:250–8. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, et al. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–62. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 85.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–3. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–43. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–39. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A. 2007;104:9301–6. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–25. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Savignac M, Mellstrom B, Naranjo JR. Calcium-dependent transcription of cytokine genes in T lymphocytes. Pflugers Arch. 2007;454:523–33. doi: 10.1007/s00424-007-0238-y. [DOI] [PubMed] [Google Scholar]
- 94.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–87. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 95.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–8. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 96.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–59. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 97.Henney CS, Bubbers JE. Antigen-T lymphocyte interactions: inhibition by cytochalasin B. J Immunol. 1973;111:85–90. [PubMed] [Google Scholar]
- 98.Holsinger LJ, Graef IA, Swat W, Chi T, Bautista DM, et al. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol. 1998;8:563–72. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- 99.Jacobelli J, Chmura SA, Buxton DB, Davis MM, Krummel MF. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat Immunol. 2004;5:531–8. doi: 10.1038/ni1065. [DOI] [PubMed] [Google Scholar]
- 100.Faure S, Salazar-Fontana LI, Semichon M, Tybulewicz VL, Bismuth G, et al. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat Immunol. 2004;5:272–9. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- 101.Gomez TS, Kumar K, Medeiros RB, Shimizu Y, Leibson PJ, Billadeau DD. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26:177–90. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeng R, Cannon JL, Abraham RT, Way M, Billadeau DD, et al. SLP-76 coordinates Nck-dependent Wiskott-Aldrich syndrome protein recruitment with Vav-1/Cdc42-dependent Wiskott-Aldrich syndrome protein activation at the T cell-APC contact site. J Immunol. 2003;171:1360–8. doi: 10.4049/jimmunol.171.3.1360. [DOI] [PubMed] [Google Scholar]
- 103.Gomez TS, Hamann MJ, McCarney S, Savoy DN, Lubking CM, et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat Immunol. 2005;6:261–70. doi: 10.1038/ni1168. [DOI] [PubMed] [Google Scholar]
- 104.Kupfer A, Swain SL, Singer SJ. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J Exp Med. 1987;165:1565–80. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Combs J, Kim SJ, Tan S, Ligon LA, Holzbaur EL, et al. Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci U S A. 2006;103:14883–8. doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 107.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 108.Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 109.Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 2002;158:1263–75. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–62. doi: 10.1038/ni1272. Peripheral microclusters, not the cSMAC, are the sites of both TCR signal initiation and maintenance. [DOI] [PubMed] [Google Scholar]
- 111.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–27. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–6. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nguyen K, Sylvain NR, Bunnell SC. T cell costimulation via the integrin VLA-4 inhibits the actin-dependent centralization of signaling microclusters containing the adaptor SLP-76. Immunity. 2008;28:810–21. doi: 10.1016/j.immuni.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 114.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–91. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 115.Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, et al. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001;15:739–50. doi: 10.1016/s1074-7613(01)00224-2. [DOI] [PubMed] [Google Scholar]
- 116.Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, et al. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang W, Irvin BJ, Trible RP, Abraham RT, Samelson LE. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol. 1999;11:943–50. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- 118.Billadeau DD, Nolz JC, Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol. 2007;7:131–43. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- 119.Menasche G, Kliche S, Bezman N, Schraven B. Regulation of T-cell antigen receptor-mediated inside-out signaling by cytosolic adapter proteins and Rap1 effector molecules. Immunol Rev. 2007;218:82–91. doi: 10.1111/j.1600-065X.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 120.Katagiri K, Hattori M, Minato N, Irie S, Takatsu K, Kinashi T. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol Cell Biol. 2000;20:1956–69. doi: 10.1128/mcb.20.6.1956-1969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sebzda E, Bracke M, Tugal T, Hogg N, Cantrell DA. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat Immunol. 2002;3:251–8. doi: 10.1038/ni765. [DOI] [PubMed] [Google Scholar]
- 122.Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJ. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol Cell Biol. 2006;26:643–53. doi: 10.1128/MCB.26.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peterson EJ, Woods ML, Dmowski SA, Derimanov G, Jordan MS, et al. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science. 2001;293:2263–5. doi: 10.1126/science.1063486. [DOI] [PubMed] [Google Scholar]
- 124.Kliche S, Breitling D, Togni M, Pusch R, Heuer K, et al. The ADAP/SKAP55 signaling module regulates T-cell receptor-mediated integrin activation through plasma membrane targeting of Rap1. Mol Cell Biol. 2006;26:7130–44. doi: 10.1128/MCB.00331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang H, McCann FE, Gordan JD, Wu X, Raab M, et al. ADAP-SLP-76 binding differentially regulates supramolecular activation cluster (SMAC) formation relative to T cell-APC conjugation. J Exp Med. 2004;200:1063–74. doi: 10.1084/jem.20040780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Menasche G, Kliche S, Chen EJ, Stradal TE, Schraven B, Koretzky G. RIAM links the ADAP/SKAP-55 signaling module to Rap1, facilitating T-cell-receptor-mediated integrin activation. Mol Cell Biol. 2007;27:4070–81. doi: 10.1128/MCB.02011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lafuente EM, van Puijenbroek AA, Krause M, Carman CV, Freeman GJ, et al. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004;7:585–95. doi: 10.1016/j.devcel.2004.07.021. RIAM is identified a key adapter in Rap1 plasma membrane localization and integrin activation in T cells. [DOI] [PubMed] [Google Scholar]
- 128.Medeiros RB, Dickey DM, Chung H, Quale AC, Nagarajan LR, et al. Protein kinase D1 and the beta 1 integrin cytoplasmic domain control beta 1 integrin function via regulation of Rap1 activation. Immunity. 2005;23:213–26. doi: 10.1016/j.immuni.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 129.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol. 2003;4:741–8. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 130.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, et al. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol. 2006;16:1796–806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 131.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–51. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 132.Pages F, Ragueneau M, Rottapel R, Truneh A, Nunes J, et al. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 1994;369:327–9. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- 133.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–4. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- 134.Huang GN, Huso DL, Bouyain S, Tu J, McCorkell KA, et al. NFAT binding and regulation of T cell activation by the cytoplasmic scaffolding Homer proteins. Science. 2008;319:476–81. doi: 10.1126/science.1151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–77. doi: 10.1016/s1074-7613(02)00323-0. CD28 costimulation increases glucose uptake and glycolysis, via a PI3K/Akt pathway, to levels exceeding that required for immediate use. [DOI] [PubMed] [Google Scholar]
- 136.Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–86. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heyeck SD, Wilcox HM, Bunnell SC, Berg LJ. Lck phosphorylates the activation loop tyrosine of the Itk kinase domain and activates Itk kinase activity. J Biol Chem. 1997;272:25401–8. doi: 10.1074/jbc.272.40.25401. [DOI] [PubMed] [Google Scholar]
- 138.Burr JS, Savage ND, Messah GE, Kimzey SL, Shaw AS, et al. Cutting edge: distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J Immunol. 2001;166:5331–5. doi: 10.4049/jimmunol.166.9.5331. [DOI] [PubMed] [Google Scholar]
- 139.Blanchet F, Cardona A, Letimier FA, Hershfield MS, Acuto O. CD28 costimulatory signal induces protein arginine methylation in T cells. J Exp Med. 2005;202:371–7. doi: 10.1084/jem.20050176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bachmaier K, Pummerer C, Shahinian A, Ionescu J, Neu N, et al. Induction of autoimmunity in the absence of CD28 costimulation. J Immunol. 1996;157:1752–7. [PubMed] [Google Scholar]
- 141.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–6. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 142.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 143.Arimura Y, Kato H, Dianzani U, Okamoto T, Kamekura S, et al. A co-stimulatory molecule on activated T cells, H4/ICOS, delivers specific signals in T(h) cells and regulates their responses. Int Immunol. 2002;14:555–66. doi: 10.1093/intimm/dxf022. [DOI] [PubMed] [Google Scholar]
- 144.Harada Y, Ohgai D, Watanabe R, Okano K, Koiwai O, et al. A single amino acid alteration in cytoplasmic domain determines IL-2 promoter activation by ligation of CD28 but not inducible costimulator (ICOS) J Exp Med. 2003;197:257–62. doi: 10.1084/jem.20021305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Suh WK, Tafuri A, Berg-Brown NN, Shahinian A, Plyte S, et al. The inducible costimulator plays the major costimulatory role in humoral immune responses in the absence of CD28. J Immunol. 2004;172:5917–23. doi: 10.4049/jimmunol.172.10.5917. [DOI] [PubMed] [Google Scholar]
- 146.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 147.Chung JY, Park YC, Ye H, Wu H. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J Cell Sci. 2002;115:679–88. doi: 10.1242/jcs.115.4.679. [DOI] [PubMed] [Google Scholar]
- 148.So T, Lee SW, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4-1BB. Cytokine Growth Factor Rev. 2008;19:253–62. doi: 10.1016/j.cytogfr.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vang T, Abrahamsen H, Myklebust S, Enserink J, Prydz H, et al. Knockdown of C-terminal Src kinase by siRNA-mediated RNA interference augments T cell receptor signaling in mature T cells. Eur J Immunol. 2004;34:2191–9. doi: 10.1002/eji.200425036. [DOI] [PubMed] [Google Scholar]
- 150.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–37. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 151.Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–54. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 152.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4:124–9. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 153.Nagaishi T, Pao L, Lin SH, Iijima H, Kaser A, et al. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity. 2006;25:769–81. doi: 10.1016/j.immuni.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 154.Yasuda T, Bundo K, Hino A, Honda K, Inoue A, et al. Dok-1 and Dok-2 are negative regulators of T cell receptor signaling. Int Immunol. 2007;19:487–95. doi: 10.1093/intimm/dxm015. [DOI] [PubMed] [Google Scholar]
- 155.Dong S, Corre B, Foulon E, Dufour E, Veillette A, et al. T cell receptor for antigen induces linker for activation of T cell-dependent activation of a negative signaling complex involving Dok-2, SHIP-1, and Grb-2. J Exp Med. 2006;203:2509–18. doi: 10.1084/jem.20060650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shui JW, Boomer JS, Han J, Xu J, Dement GA, et al. Hematopoietic progenitor kinase 1 negatively regulates T cell receptor signaling and T cell-mediated immune responses. Nat Immunol. 2007;8:84–91. doi: 10.1038/ni1416. [DOI] [PubMed] [Google Scholar]
- 157.Di Bartolo V, Montagne B, Salek M, Jungwirth B, Carrette F, et al. A novel pathway down-modulating T cell activation involves HPK-1-dependent recruitment of 14-3-3 proteins on SLP-76. J Exp Med. 2007;204:681–91. doi: 10.1084/jem.20062066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carpino N, Turner S, Mekala D, Takahashi Y, Zang H, et al. Regulation of ZAP-70 activation and TCR signaling by two related proteins, Sts-1 and Sts-2. Immunity. 2004;20:37–46. doi: 10.1016/s1074-7613(03)00351-0. [DOI] [PubMed] [Google Scholar]
- 159.Mikhailik A, Ford B, Keller J, Chen Y, Nassar N, Carpino N. A phosphatase activity of Sts-1 contributes to the suppression of TCR signaling. Mol Cell. 2007;27:486–97. doi: 10.1016/j.molcel.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Agrawal R, Carpino N, Tsygankov A. TULA proteins regulate activity of the protein tyrosine kinase Syk. J Cell Biochem. 2008;104:953–64. doi: 10.1002/jcb.21678. [DOI] [PubMed] [Google Scholar]
- 161.Feshchenko EA, Smirnova EV, Swaminathan G, Teckchandani AM, Agrawal R, et al. TULA: an SH3- and UBA-containing protein that binds to c-Cbl and ubiquitin. Oncogene. 2004;23:4690–706. doi: 10.1038/sj.onc.1207627. [DOI] [PubMed] [Google Scholar]
- 162.Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–6. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 163.Naramura M, Jang IK, Kole H, Huang F, Haines D, Gu H. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat Immunol. 2002;3:1192–9. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- 164.Murphy MA, Schnall RG, Venter DJ, Barnett L, Bertoncello I, et al. Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol Cell Biol. 1998;18:4872–82. doi: 10.1128/mcb.18.8.4872. Three papers demonstrating autoimmunity in Cbl−/− mice, and identifying Cbl family members as critical negative regulators of T cell activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Balagopalan L, Barr VA, Sommers CL, Barda-Saad M, Goyal A, et al. c-Cbl-mediated regulation of LAT-nucleated signaling complexes. Mol Cell Biol. 2007;27:8622–36. doi: 10.1128/MCB.00467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 167.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 168.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 169.Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]