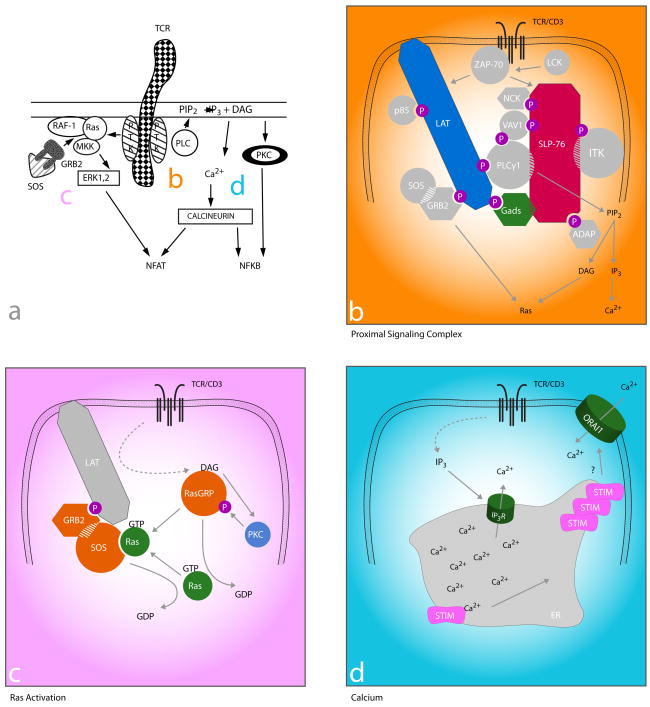
Figure 1. TCR proximal signaling then and now: filling in the gaps.
Then: a. TCR signaling mid-1990s (adapted from 2). Ligation of the TCR/CD3 results in activation of src and syk family PTKs associated with the intracellular CD3 domains that then activate PLC and Ras-dependent pathways.
Now: b–d. Current understanding of how the TCR couples to PLC activation (b), Ras (c), and the molecular basis for Ca2+ influx (d). b. The link between PTKs and downstream pathways (including those in c and d) is a multi-molecular signaling complex nucleated by the adapter proteins LAT, Gads, and SLP-76. Lck activates ZAP-70 to phosphorylate (p) tyrosine residues on LAT, which then recruits Gads and its constitutive binding partner SLP-76. ZAP-70-mediated phosphorylation of SLP-76 results in the recruitment of multiple SH2 domain-containing effector molecules (circles) and adapter proteins (octogons). SH3 domains (hatching) also link effectors to adapters and contribute to stabilization of the complex. c. Activation of Ras involves two Ras GEFs (Sos and RasGRP) in a positive feedback loop. TCR-induced production of DAG (see b) results in the membrane recruitment of RasGRP, where it is phosphorylated and activated by PKC. RasGRP then facilitates the removal of GDP from Ras, which can then bind GTP and become activated. GTP-bound Ras then binds Sos (bound constitutively to GRB2, which is inducibly recruited to LAT), increasing its GEF activity resulting in a positive feedback loop and robust Ras activity. d. The link between depletion of ER Ca2+stores and CRAC channel activation is Stim. TCR-induced IP3 production (see b) results in the activation of IP3 receptors (IP3R), which release Ca2+ from the ER into the cytoplasm. Stim contains paired EF hands within the ER lumen, which bind Ca2+. Upon depletion of ER stores, Ca2+-free Stim molecules cluster and are thought to move to areas of ER/plasma membrane proximity where they co-localize with CRAC channels (the pore-forming subunit has been identified as Orai1) and contribute to their activation and the subsequent influx of Ca2+.