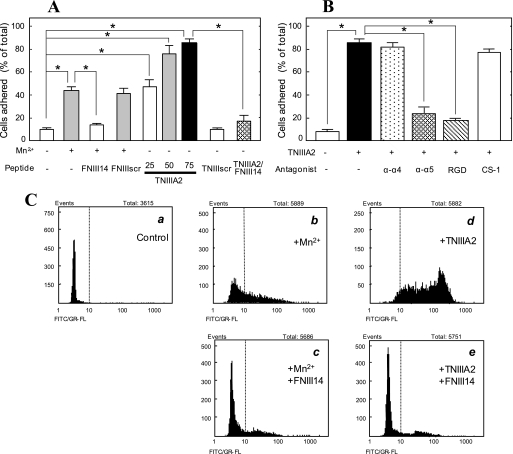
FIGURE 1.
Adhesion of K562 cells to FN through β1-integrin activation. A, K562 cell suspension with or without Mn2+ (0.5 mm), FNIII14 or its control FNIII14scr (100 μg/ml), TNIIIA2 (25–75 μg/ml) or its control TNIIIA2scr (100 μg/ml), or a combination of these was seeded in a 96-well plate coated with the FN (5 μg/ml). B, K562 cell suspension with or without TNIIIA2 (75 μg/ml) was seeded in the FN-coated plate in the presence of the function-blocking mAb to integrin α4 or α5 subunit (20 μg/ml) or RGD or CS-1 peptide (200 μg/ml). An adhesion assay was performed in A and B, as described under “Experimental Procedures.” The ordinates show the percentage of the number of adhered cells, relative to the total number of cells seeded into the well. Each point represents the mean ± S.E. of triplicate determinations. Data are representative of three individual experiments. *, p < 0.01 compared with control. C, flow cytometric analysis of β1-integrin activation in K562 cells. Cells suspended in serum-free medium were incubated at 37 °C for 30 min with occasional shaking under the following conditions. a, none; b, Mn2+ (0.5 mm); c, Mn2+ (0.5 mm) plus FNIII14 (100 μg/ml); d, TNIIIA2 (50 μg/ml); e, TNIIIA2 (50 μg/ml) plus FNIII14 (100 μg/ml). Flow cytometric analyses were performed using the FITC-labeled normal mouse IgG (a) or FITC-labeled anti-β1 integrin mAb, AG89 (b–e), as described under “Experimental Procedures.” Data shown are representative of three individual experiments.