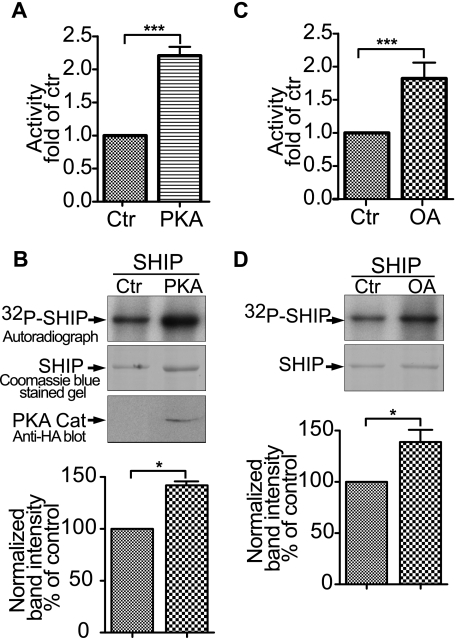
FIGURE 5.
Phosphorylation regulates SHIP1 activity in HEK-293 cells. A, cells were transfected with SHIP1 with or without PKA catalytic subunit for 40 h. Recombinant SHIP1 was immunopurified, the protein concentration was determined, and the activity of 10 ng of SHIP1 was measured. The effect of PKA on activity was significant. ***, p < 0.001 (n = 6). B, upper panel presents an autoradiograph showing the effect of co-expression of the PKA catalytic subunit on the phosphorylation of SHIP1. An immunoblot with the anti-hemagglutinin antibody shows the expression of hemagglutinin-tagged PKA catalytic subunit. The lower panel presents the amount of 32P incorporated into SHIP1 based on band intensity normalized for protein expression. The data appear below the respective band and are expressed as the percentages of control (Ctr). *, p < 0.05 (n = 3). C, HEK-293 cells were transfected with SHIP1 for 40 h and cells were treated with or without 100 nm okadaic acid (OA) for 2 h. SHIP1 was immunopurified and assayed for activity. ***, p < 0.001 (n = 6). D, upper panel presents an autoradiograph showing the effect of okadaic acid on phosphorylation of SHIP1. The lower panel presents the amount of 32P incorporated into SHIP1 based on band intensity normalized for protein expression. *, p < 0.05 (n = 3).