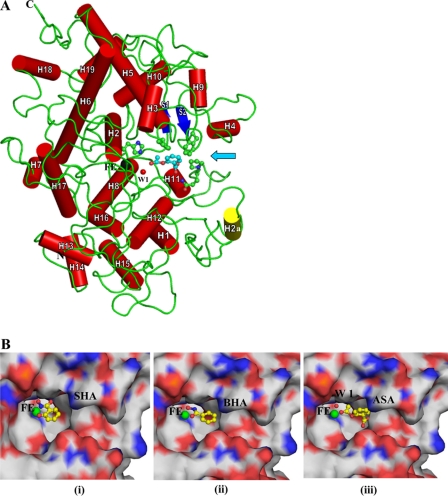
FIGURE 3.
A, the overall folding of the protein shown as a schematic, where α-helices are indicated as cylinders (red) and numbered. H2a is a unique α-helix (yellow) present only in LPO but absent in MPO. The two anti-parallel β-strands are drawn as arrows (blue). For sake of clarity, only ASA (magenta) is shown in the substrate binding site. Heme iron (Fe) is shown in black, whereas conserved heme water molecule (W-1) is indicated in red. The heme moiety is not shown to avoid crowding. Some of the residues, His-109, Phe-113, Phe-380, Gln-423, and Pro-424, of the substrate binding site are shown in ball and stick representation. An arrow indicates the entrance to the substrate binding site. B, close-up view of ligands bound at the distal site ASA (i), SHA (ii), and BHA (iii). The heme iron atom is indicated in green. The ligands are represented as ball and stick models.