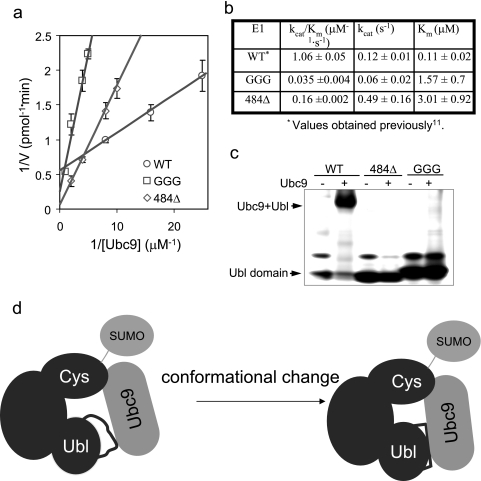
FIGURE 6.
E1 mutants are deficient in recruiting E2. a, double-reciprocal plot of the steady-state kinetics of E1-catalyzed trans-thiolation in overall conjugation reactions. Initial rates for SUMO-RanGAP1 conjugation were determined under E1-limiting conditions (see “Experimental Procedures”). E1 concentration was 1 μm for the GGG mutant and 400 nm for the 484Δ mutant. Ubc9 concentrations are indicated in the figure. Because of its lower activity, higher concentrations of Ubc9 were used for GGG and 484Δ than for the wild-type protein (WT) to reliably measure the initial rates. b, kinetic rate constants extracted from the plots for the mutant E1 proteins along with the values of the wild type reported previously (11). The kinetic constants are normalized to the percentage of the E1 enzyme that is active, judged by the percentage of E1 that can form thioester conjugates with SUMO. c, gel electrophoresis to detect the Ubc9·Ubl complex. Approximately 40 μg of Ubc9 was incubated (1 h, room temperature) with ∼6 μg of either the wild-type or one of the mutant Ubl domains. Proteins were separated on nondenaturing 8–16% gel and stained with SimpleBlue. Because of the charge property, Ubc9 did not run into the gel. d, schematic illustration of the conformational transition during E1 and E2 association.