Table 3.
Stereoselective Synthesis of Trisubstituted α-Hydroxycyclopropyl Carbinols
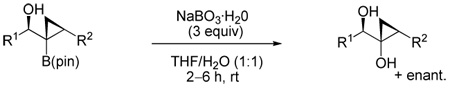 | |||
|---|---|---|---|
| entry | cyclopropyl boronate ester |
α-hydroxy cyclopropyl carbinol |
Isolated yield (%)a |
| 1 |  |
 |
89b |
| 2 |  |
 |
93b |
| 3 |  |
 |
91 |
| 4 | 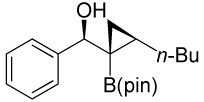 |
 |
90 |
| 5 |  |
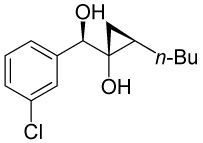 |
86 |
| 6 | 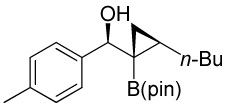 |
 |
88 |
| 7 |  |
 |
75b |
| 8 |  |
 |
80 |
Only one diastereomer detected by 1H NMR.
Syn stereochemistry of α-hydroxycyclopropyl carbinols established by single crystal X-ray diffraction.
