Table 1.
Reaction of a variety of allylic carbonates with BocNH2.
| entrya | product | b:lb | yield (%)c | ee (%)d |
|---|---|---|---|---|
| 1e | 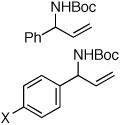 |
94:6 | 85 | 99 |
| 2f | X = CF3 | 81:19 | 65 | 99 |
| 3f | X = Br | 91:9 | 79 | 99 |
| 4 | X = OMe | 99:1 | 85 | 99 |
| 5 | 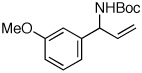 |
95:5 | 81 | 99 |
| 6 | 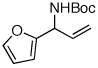 |
83:17 | 50 | 98 |
| 7 | 89:11 | 79 | 95 | |
| 8 | 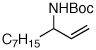 |
85:15 | 65 | 97 |
| 9 | 83:17 | 61 | 97 | |
| 10f,g | 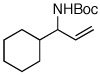 |
55:45 | 25 | 99 |
General conditions: 0.7 mmol BocNH2, 0.5 mmol carbonate, 0.02 mmol 1d, and 10 µL dodecane in 0.5 mL ether. Results are an average of two runs.
Determined by NMR analysis of the crude reaction mixture or isolation.
Isolated yield of branched product.
Determined by chiral HPLC analysis, see Supporting Information for details.
THF was used as solvent.
K3PO4 (0.25 mmol) was added.
Reaction was run at 30 °C.
