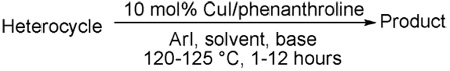
Table 2.
Electron-poor Heterocycle Arylationa
 | ||||
|---|---|---|---|---|
| entry | heterocycle | aryl halide/base | product | yield, % |
| 1b | 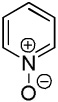 |
C6H5I/tBuOLi | 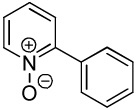 |
58 |
| 2 | 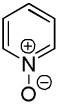 |
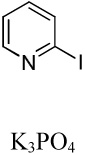 |
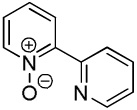 |
41 |
| 3 | 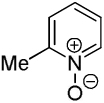 |
C6H5I/tBuOLi | 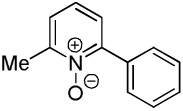 |
43 |
| 4 | 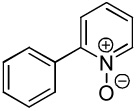 |
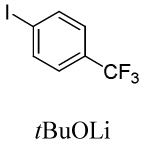 |
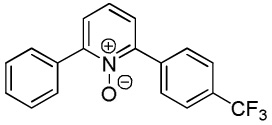 |
80 |
| 5 | 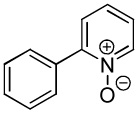 |
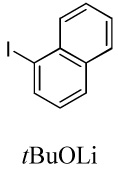 |
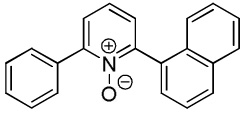 |
91 |
| 6 | 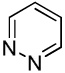 |
C6H5I/Et3COLi | 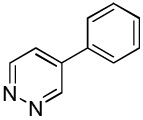 |
60 |
| 7 | 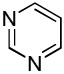 |
C6H5I/Et3COLi | 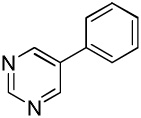 |
31 |
Copper (I) iodide (0.1 mmol), phenanthroline (0.1 mmol), aryl halide (1–2 mmol), heterocycle (1–2 mmol), base (1.7–2 mmol), DMF or DMPU solvent (0.5–0.6 mL). Yields are isolated yields. See the Supporting Information for details.
2,6-Diphenylpyridine oxide also isolated (20%).
