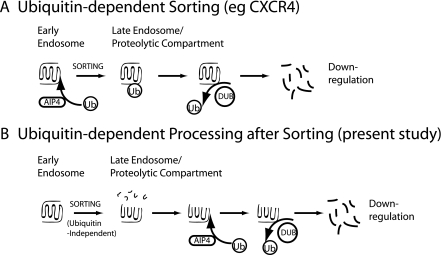
FIGURE 6.
Proposed model for the postsorting function of AIP4 in controlling DOR down-regulation. A, depiction of the current view of AIP4-dependent regulation of GPCR proteolysis, based on previous studies of the CXCR4 receptor (3, 24). Ubiquitination acts as a sorting determinant that is required for delivery of internalized receptors to the late endosome/lysosome pathway, and receptor proteolysis follows. Receptors presumably undergo deubiquitination, as indicated, which is not specifically required for lysosomal sorting of receptors but is thought to occur generally after sorting is complete to prevent depletion of free cytoplasmic ubiquitin (Ub) pools (36). B, the currently proposed function of AIP4-dependent ubiquitination in regulating later proteolytic processing of DOR. Endocytic sorting of the DOR into the late endosome/lysosome pathway does not require receptor ubiquitination, in contrast to that of the CXCR4 receptor, as indicated by the ability of the lysyl-mutant DORs to undergo complete proteolytic destruction at a rate similar to that of wild type receptors. Wild type receptors are also sorted into the late endosome/lysosome pathway irrespective of their ubiquitination state, as indicated by LAMP1/2 colocalization and initial proteolytic fragmentation detected biochemically. Subsequent proteolytic processing required to destroy the hydrophobic core of the receptor is specifically regulated by both ubiquitination/deubiquitination, as indicated by the pronounced inhibition produced by disrupting either ubiquitin ligase (AIP4) or hydrolase (DUB) activity on down-regulation of wild type but not lysyl-mutant receptors detected by radioligand binding. The critical distinctions from model A are 1) that ubiquitination affects proteolytic processing of the wild type DOR clearly after receptor sorting to a lysosomal fate and the occurrence of initial proteolytic cleavage events, and 2) that later proteolytic processing of receptors is specifically regulated by both receptor ubiquitination and deubiquitination.