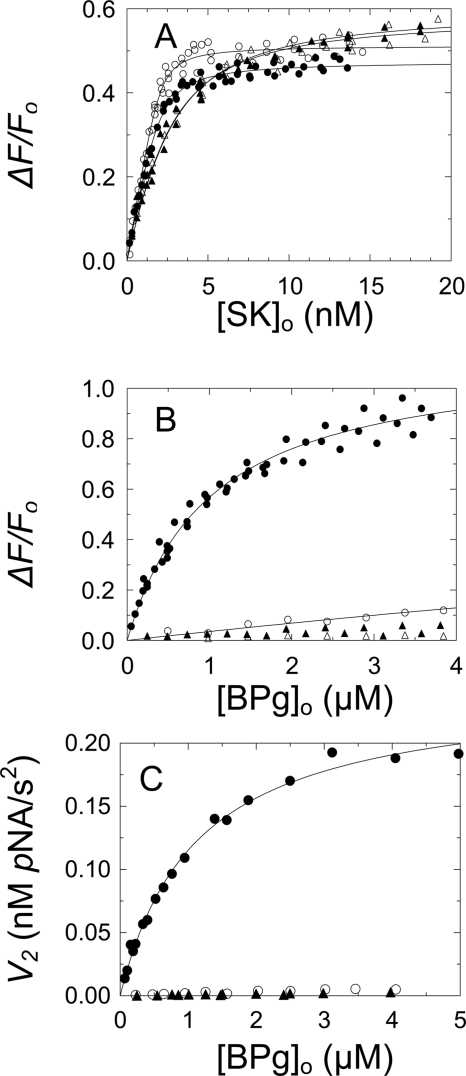
FIGURE 3.
Ternary complex formation among SK or Met-SK(K256A/K257A), [TMR]FPR-Pm, and BPg and kinetics of BPg activation by SK·Pm. A, fractional change in fluorescence intensity (ΔF/Fo) as a function of total native SK concentration (●) or total Met-SK(K256A/K257A) concentration ([SK]o) (○) in the absence of 6-AHA. Titrations with native SK (▴) or Met-SK(K256A/K257A) (▵) in the presence of 40 mm 6-AHA. Solid lines represent the nonlinear least-squares fits of the quadratic binding equation with the parameters given under ”Results.“ Fluorescence titrations were performed and analyzed as described under ”Experimental Procedures.“ B, fractional change in fluorescence intensity (ΔF/Fo) as a function of total BPg concentration ([BPg]o) for 35 nm [TMR]FPR-Pm·native SK complex formed in the presence of 98 nm SK in the absence of 6-AHA (●) and in the presence of 230 nm SK and 40 mm 6-AHA (○). Titrations of the Met-SK(K256A/K257A)·[TMR]FPR-Pm complex formed in the presence of 98 nm Met-SK(K256A/K257A) in the absence of 6-AHA (▴) and in the presence of 230 nm Met-SK(K256A/K257A) and 40 mm 6-AHA (▵). Solid lines, least-squares fit of the quadratic binding equation to the [TMR]FPR-Pm·native SK titration (●) with the parameters given under ”Results“ and the fit of the [TMR]FPR-Pm·native SK titration in the presence of 40 mm 6-AHA (○), assuming the same maximum fluorescence change and a KD value of 31 μm. Fluorescence titrations were performed and analyzed as described under ”Experimental Procedures.“ C, initial velocity (v2) of BPg activation by 0.1 nm native SK·Pm complex as a function of total BPg concentration ([BPg]o) in the absence (●) and presence of 40 mm 6-AHA (○) and initial rates of BPg activation by 0.09 nm Met-SK(K256A/K257A)·Pm complex in the absence of 6-AHA (▴). The solid line represents the fit by the Michaelis-Menten equation with the parameters given under ”Results.“ Initial velocities of BPg activation were measured and analyzed as described under ”Experimental Procedures.“