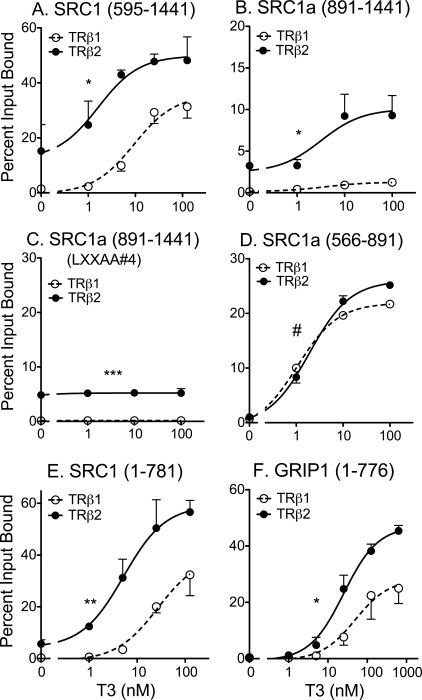
FIGURE 4.
Identification of three domains on the p160 coactivators required for preferential recruitment by TRβ2. A, preferential TRβ2 binding to an SRC1a-(595–1441) construct containing the central LXXLL motifs, the Gln-rich domain, and the C-terminal LXXLL motif. Protocol was as described in the legend to Fig. 3B. B, preferential TRβ2 binding to an SRC1a-(891–1441) construct containing the central LXXLL motifs, the Gln-rich domain, and the C-terminal LXXLL motif. Protocol was as described in the legend to Fig. 3B, except a GST-SRC1-(891–1441) construct was incubated with 35S-radiolabeled full-length TRβ1 or TRβ2, as indicated. Percent bound is presented, normalized to TRβ2 levels. C, preferential, hormone-independent TRβ2 binding to an SRC1a-(891–1441) construct containing the Gln-rich domain but lacking the C-terminal LXXLL motif. Protocol was as in panel B, except the C-terminal LXXLL in the GST-SRC1-(891–1441) construct was mutated to LXXAA. D, equal TRβ2 and TRβ1 binding to an SRC1a-(566–891) construct limited to the central LXXLL motifs. Protocol was as in panel B. E, preferential TRβ2 binding to an SRC1a-(1–781) construct containing the N-terminal and central LXXLL domains, but lacking the Gln-rich region. Protocol was as in panel A. F, preferential TRβ2 binding to a GRIP1-(1–776) construct containing the N-terminal and central LXXLL domains, but lacking the Gln-rich region. Protocol was as in panel A. Statistical confidence symbols are as described in the legend to Fig. 3.