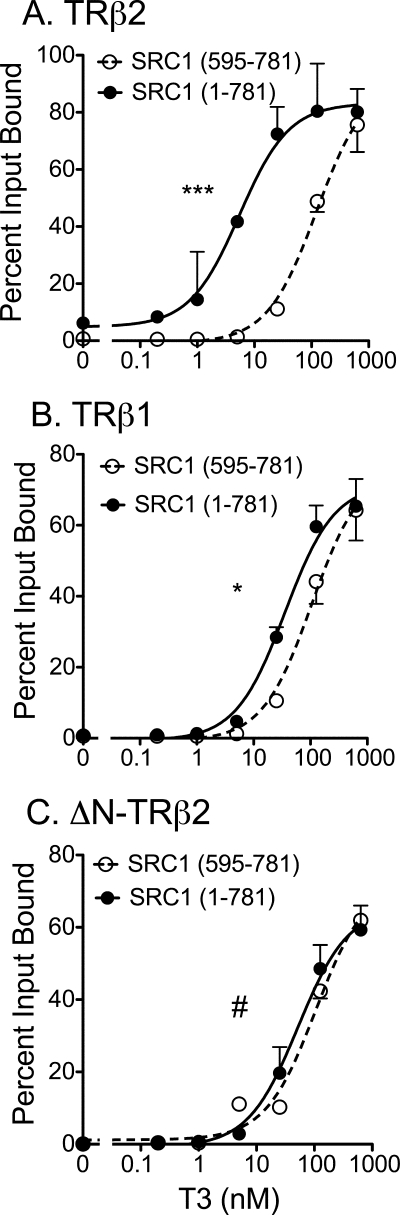
FIGURE 5.
Positive contribution of the N-terminal domain of TRβ2 to recruitment of SRC1a. 35S-Radiolabeled versions of SRC1 (codons 1–781) and SRC1 (codons 595–781) were mixed together and incubated with each immobilized GST-TRβ construct over a range of T3 concentrations, as described in the legend to Fig. 4E. The SRC1-(1–781) and SRC1-(595–781) coactivator proteins bound by each receptor were eluted, resolved from one another by SDS-PAGE, quantified, and are presented as the percent of their input values. A, strong preference of TRβ2 for SRC1 constructs retaining the N-terminal domain. B, minor preference of TRβ1 for SRC1 constructs retaining the N-terminal domain. C, lack of preference of ΔN-TRβ2 for SRC1 constructs retaining the N-terminal domain. Statistical confidence symbols are as described in the legend to Fig. 3.