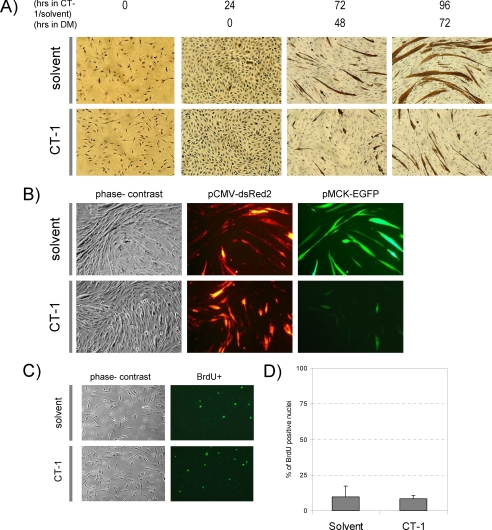
FIGURE 1.
CT-1 represses myogenic differentiation. A, C2C12 cells were seeded onto cell culture plates at equal density and maintained in CT-1 (10 ng/ml) or solvent containing growth medium (GM) or differentiation medium (DM) for the indicated time period. The cells were fixed and stained for muscle myosin heavy chain (MyHC) detection by immunochemistry. The photomicrographs are representative fields in each condition. B, C2C12 cells were plated at equal density and transfected with pCMV-deRed2 and pMCK-eGFP constructs. The transfected cells were maintained in CT-1 (10 ng/ml) or solvent containing DM for 72 h to induce myotube formation. The cell morphology was recorded by phase-contrast microscopy and transfected cells were monitored by the red fluorescence signal. MCK promoter activity was assessed by the green fluorescence signal. C, C2C12 cells were maintained in DM for 72 h and CT-1 (10 ng/ml) or solvent was added every 24 h. After 72 h in low serum conditions cells were incubated with 100 μm of bromodeoxyuridine for 1 h. Cells were then fixed with 70% ethanol and then incubated with 2N HCl to denature the DNA. The cells were then blocked with 10% goat serum prior to incubation with bromodeoxyuridine primary antibody. The cells were then washed with 1x PBS-T and incubated with secondary antibody conjugated to FITC. The cells were washed with 1x PBS-T and mounted using fluorescence mounting media and viewed under a fluorescence microscope. D, the average of percentage of bromodeoxyuridine positive nuclei over total nuclei in 12 individual fields per condition was calculated and graphed. (error = standard variation).