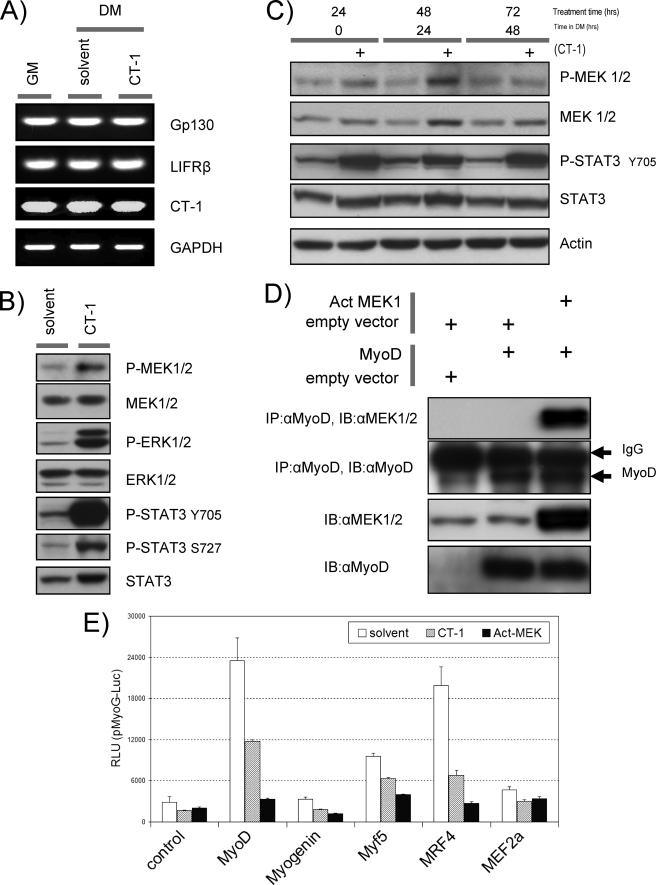
FIGURE 4.
Trans-activation properties of the MRFs are repressed by CT-1. A, total RNA was isolated from C2C12 cells in GM (lane 1), DM with solvent (48 h) (lane 2), and DM with CT-1 (10 ng/ml) (48 h) (lane 3) and subjected to semi-quantitative reverse transcription-RCP analysis with indicated gene specific primer pairs. reverse transcription-PCR amplified DNA was separated in a TAE/agarose-gel, and ethidium bromide stained DNA was visualized by UV irradiation. GAPDH serves as an internal loading control. B, C2C12 cells were plated at equal density and kept in DM for 16 h. CT-1 (10 ng/ml) or equal volume of the solvent was added to the media. The cells were harvested after 20min of CT-1/solvent addition. Total protein samples were subjected to Western blotting analysis to estimate the levels of indicated proteins. C, Western blotting analysis was performed as described above. However, the cells were maintained in DM with CT-1 or solvent for indicated time periods. D, C3H10T1/2 cells were transfected with combinations of the indicated constructs. Total protein samples were extracted from the cells maintained in DM. Exogenous-expression of MyoD and an activated form of MEK1 was confirmed by immuno-blotting (IB) (10 μg loading) with the specific antibodies. An immuno-precipitation (IP) analysis was performed with the total protein extract (250 μg) with MyoD antibody (mouse) and proteinG conjugated beads. Precipitated immuno-complex were eluted off the proteinG beads and subjected for an immunoblotting with MEK antibody (Rabbit). Equal amount of IgG loading was monitored with MyoD immuno-blotting with MyoD specific antibody (Rabbit). E, C2C12 cells were transfected with the indicated expression constructs or its empty vector (1 μg) and myoG promoter- (pMyoG-Luc) promoter-Luciferase reporter construct (0.5 μg). In addition, an activated form of MEK1 expression vector (1 μg) or its empty vector (for CT-1 and solvent) was included. To monitor transfection efficiency, a pCMV-β-Gal construct was also included (0.3 μg). After transfection, the cells were maintained in DM containing CT-1 (10 ng/ml) or its solvent for 16 h. The cells were harvested and subjected to luciferase assay and β-Galactosidase assay.