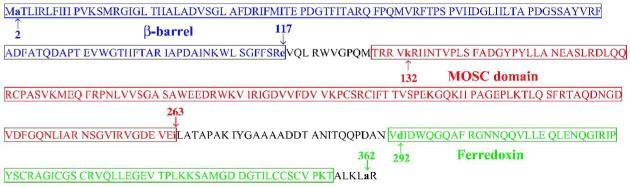
Fig. 6.
Sequence and predicted domain architecture of YcbX protein. The predicted domains (indicated by colored boxes) are β-barrel (M1-E117) (blue), MOSC (T128-I263) (red), and Ferredoxin (V291-T363) (green). The Figure also indicates (by small arrows) the endpoints of the domain-specific ycbX deletions created in this study. In strain NR16261 (ΔycbX[Δ(β-barrel)]) β-barrel amino acids A2 through E117 (endpoints marked by blue arrows) are replaced by the stuffer peptide IPGIRRPAVRSSYSLESIGTSKQLQPTQ. In strain NR16259 (ΔycbX[Δ(MOSC)]) MOSC-domain amino acids K132 to I263 (red arrows) are replaced by the polypeptide IPGIRRPAVRSSYSLESIGTSKQLQPTL. In strain NR16258 (ΔycbX[Δ(Ferredoxin)]) Ferredoxin-domain amino acids D292 through A362 (green arrows) are replaced by polypeptide IPGIRRPAVRSSYSLESIGTSKQLQPT. In strain NR16262 (ΔycbX), carrying a complete deletion of the ycbX gene, the polypeptide MIPGIRRPAVRSSYSLESIGTSKQLQPTRR replaces the YcbX protein. See text and Experimental procedures for further details.