Abstract
Purpose
Neuroblastoma is a childhood cancer of the sympathetic nervous system and many patients present with high risk disease. Risk stratification, based on pathology and tumor-derived biomarkers, has improved prediction of clinical outcomes, but overall survival rates remain unfavorable and new therapeutic targets are needed. Some studies suggest a link between interleukin-6 and more aggressive behavior in neuroblastoma tumor cells. Therefore, we examined the impact of two IL-6 single nucleotide polymorphisms (SNP) on neuroblastoma disease progression.
Experimental design
DNA samples from 96 high risk neuroblastoma patients were screened for two SNP that are known to regulate the serum levels of IL-6 and the soluble IL-6 receptor (IL-6R), rs1800795 and rs8192284 respectively. The genotype for each SNP was determined in a blinded fashion and independent statistical analysis was performed to determine SNP-related event free survival (EFS) and overall survival (OS) rates.
Results
The rs1800795 IL-6 promoter SNP is an independent prognostic factor for EFS and OS in -high risk neuroblastoma patients. In contrast, the rs8192284 IL-6 receptor SNP revealed no prognostic value.
Conclusions
The rs1800795 SNP (-174 IL-6 (G>C) represents a novel and independent prognostic marker for both EFS and OS in high risk neuroblastoma. Since the rs1800795 SNP (-174 IL-6 (G>C) has been shown to correlate with production of IL-6, this cytokine may represent a target for development of new therapies in neuroblastoma.
Introduction
Neuroblastoma is the third most common solid tumor in children less than 19 years of age. While neuroblastoma represents less than 10% of all pediatric neoplasms, it is responsible for approximately 15% of all pediatric oncology-related deaths 1. One of several factors that contribute to poor clinical outcome in neuroblastoma is that over 40% of children with neuroblastoma are categorized as high risk based on the International Neuroblastoma Staging System (INSS) 1. Despite aggressive intervention, including chemotherapy, surgery, radiation and myeloablative therapy and stem cell rescue, the overall survival rate remains near 30% 1. Bone is a common site of neuroblastoma metastases, occurring in over half of newly diagnosed patients, and bone metastasis correlates with poor clinical outcomes in neuroblastoma 2.
Metastatic colonization of secondary organs is influenced by interactions between circulating tumor cells and the tissue microenvironments. The bone microenvironment can be a particularly fertile soil for tumor cells due to large repositories of various growth factors. Chemokines generated by these interactions can facilitate not only homing of additional tumor cells but also expanded proliferation and survival of local tumor cell populations. Interleukin 6 (IL-6), a pro-inflammatory cytokine, plays an active role in neoplasia, bone metabolism and iron homeostasis 3, 4. A role for IL-6 in the disease progression of several neoplasms such as multiple myeloma 5, colon cancer 6, renal cancer 7, Hodgkin’s disease 8, non-Hodgkin’s lymphoma 9, prostate cancer 10, melanoma 11 and breast cancer 12, 13, has been well documented. Recently, it has been shown that peripheral blood IL-6 levels correlated with disease extent and progression of neuroblastoma 14.
Once in the bone microenvironment, neuroblastoma forms predominantly osteolytic tumors15. Recent data suggests that paracrine IL-6 signaling events are involved causing the morbidity associated with neuroblastoma bone metastasis 16. Bone marrow fibroblasts (i.e. mesenchymal stem cells (MSC)) secrete elevated amounts of IL-6 in the presence of neuroblastoma cells 17, 18, which in turn elevates osteoclast activity and promotes neuroblastoma tumor cell expansion through activation of STAT3 and mitogen-activated protein kinases (MAPK) p44Erk1 and p42Erk219.
Independent studies from our group found that hormone-responsive breast cancer cells display a similar molecular profile to neuroblastoma cells following paracrine IL-6 stimulation 15, 20, and that breast cancer, like neuroblastoma, has a strong predilection to metastasize to bone 2, 15, 21, 22. While a link between IL-6 and organ-specific metastasis has yet to be fully elucidated, there exists a direct correlation between the -174 IL-6 (G>C) SNP and clinical outcome in breast cancer. Women with hormone-responsive breast cancer and homozygous “G/G” at the -174 IL-6 (G>C) SNP displayed significantly lower rates of disease free survival (DFS) (P-value < 0.003) and overall survival (OS) (P-value < 0.001) 12.
Given the evidence that elevated levels of circulating IL-6 is a marker of poor prognosis in various cancers, including neuroblastoma, and the role of IL-6 in the neuroblastoma tumor microenvironment; we postulated that a correlation between clinical outcome and IL-6 SNP status may also exist in neuroblastoma patients. Data from this study suggests that increased IL-6 signaling is associated with inferior clinical outcomes in patients with high-risk neuroblastoma patients. This data also suggests that IL-6 may represent a promising extracellular target for new therapies in neuroblastoma.
Materials and Methods
Patient Samples
Ninety-six high risk neuroblastoma DNA specimens collected from blood at the time of diagnosis, with known clinical characteristics and outcome data, were randomly selected from the Neuroblastoma Virtual Tumor Bank. All patients met criteria for COG neuroblastoma high risk group and were enrolled on ANBL00B1. They were subsequently treated on a variety of COG high risk protocol. The patients in this study were enrolled on a trial between June 2, 1994 and January 19, 2007. Inclusion criteria were fulfillment of high risk definition as defined by COG risk grouping at the time of diagnosis, availability of DNA from peripheral blood, and either occurrence of an event, or ≥ 3 years of follow-up time.
Biologic Studies
DNA samples were screened for two SNPs, one of which regulates expression of soluble IL-6 and the other SNP impacts the level of soluble IL-6 receptor (sIL-6R). The SNP in the IL-6 gene promoter -174 IL-6 (G>C) (rs1800795) is known to alter expression of IL-6 following inflammatory stimulus 23, 24. The D358A IL-6 SNP (rs8192284) creates an amino acid substitution within the extracellular cleavage domain of the IL-6 receptor (IL-6R) that influences sIL-6R serum levels 24. We employed restriction fragment length polymorphism (RFLP) mapping to identify each patient as (G/G), (G/C), or (C/C) at the -174 IL-6 (G>C) SNP and (C/C), (A/C), or (A/A) at the D358A IL-6R SNP. The RFLP mapping strategy was previously described by DeMichele, et al. for the -174 IL-6 (G>C) SNP 12 A similar approach was used to assess the SNP genotype for the D358A IL-6R SNP. Both methods were PCR-based and used primers that flank the SNP locus to produce an amplicon of 305 and 524 base pairs, respectively. DNA (0.1 μg) from high risk neuroblastoma patients was used as a template, and PCR amplicons were generated following 35 cycles consisting of: melting 95°C for 2 minutes; annealing 72°C for 1 minute; extension 58°C for 1 minute. The PCR products were gel purified on 2% agarose and digested with the DNA restriction endonuclease Nla-III (-174 IL-6 (G>C) SNP) or Hind-III (D358A IL-6R SNP). The predicted band sizes for the -174 IL-6 (G>C) SNP genotypes following Nla-III digestion were (G/G) = 230bp; (G/C) = 230bp plus 121/109bp; and (C/C) = 121/109bp, and the predicted band sizes following Hind-III digestion for the D358A IL-6R SNP genotypes were (A/A) = 413; (A/C) = 413bp plus 231/182bp; (C/C) = 231/182bp (Figure 1). The DNA primer sequences utilized were: “-174 IL-6 (G > C) ” forward: ATGCCAAGTGCTGAGTCACTA, reverse: TCGAGGGCAGAATGAGCCTC, and “ D358AIL-6R” forward: GGCGCTCAGAAACCCTGAGCT, reverse: TGTGTGTGTTGTGGTGTGTGC. Each RFLP analysis included an internal positive control to verify endonuclease activity, which gave rise to products of 75bp for the -174 IL-6 (G>C) SNP and 111bp for the D358A IL-6R SNP (Figure 1). Neuroblastoma cell lines (SK-N-AS, SK-N-DZ, SK-N-FI, SK-N-SH) used in RFLP to compare with study samples (Figure 1) were purchased from American Type Culture Collection (ATCC). These cell lines originated from patients with bone marrow metastases.
Figure 1.
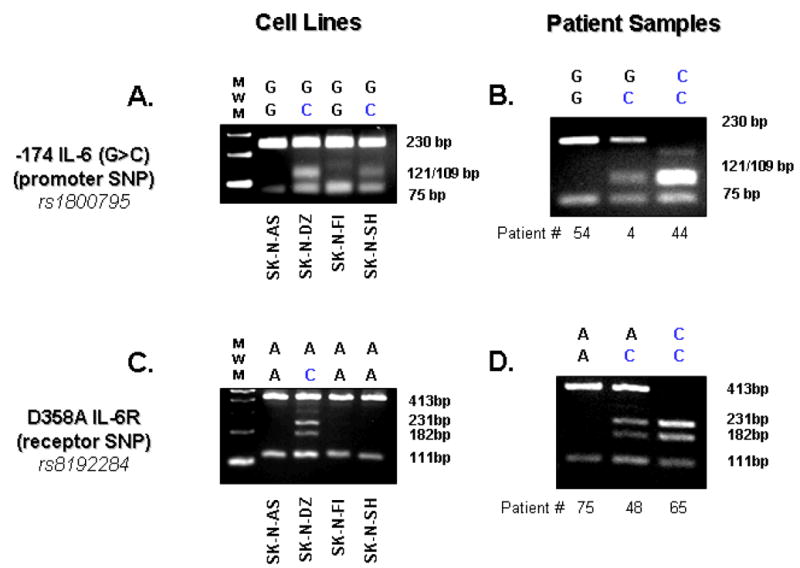
Restriction Fragment Length Polymorphism (RFLP) analysis of human neuroblastoma (NBL) cell lines and patient samples. RFLP analysis was used to identify the genotypes of the rs1800795 SNP (-174 IL-6 (G>C)) in (A) human NBL cell lines and (B) high risk NBL patient DNA samples, and in a similar fashion, the genotypes of the rs8192284 SNP (D358A IL-6R) in (C) human NBL cell lines and (D) high risk NBL patient DNA samples were also characterized. See Table 2 for full genotype analysis.
Statistical Analysis
A sample size of 70 subjects would be required to detect a 30% difference in three year-event free survival (EFS: 20% to 50%) or overall survival (OS: 30% to 60%) for the genotype G/G compared to G/C or C/C, respectively, in a logrank test with an alpha of 0.05 and 80% power. It is expected that the percentages of each genotype within the general population are as follows: 65% of the -174 IL6 (G>C) genotypes are G/C or C/C and 35% are G/G 21–24. Therefore, it was estimated that a total of 100 patients would be required to provide about 35 patients with genotype G/G, with the balance of about 65 patients of genotypes G/C or C/C.
Fisher’s exact test was used to test for a) associations of the existence of polymorphisms versus each other factor; and, b) differences in the proportion of “G/G” in the -174 IL6 SNP for the general Caucasian population versus the NB cohort. Event-free survival (EFS) time was calculated from the time of enrollment on the front-line or biologic study until the time of the first occurrence of relapse, progressive disease, secondary malignancy, or death, or until the time of last contact if no event occurred. Overall survival (OS) time was calculated from the time of enrollment on study until the time of the death, or until last contact with patient. The median follow-up time for patients alive without an event was 4.9 years (range: 3.0 to 11.4 years). The methods of Kaplan-Meier were used to generate survival curves, and curves were compared using a logrank test. EFS and OS are presented as the estimate +/− the standard error, with standard errors calculated per the methods of Peto25. The Cox proportional hazards regression model was used to test for the independent predictive ability of the existence of polymorphisms after adjustment for other significant factors. P-values <0.05 were considered statistically significant.
A secondary analysis was performed comparing G/G vs (G/C or C/C) of the promoter -174 IL-6 (G>C) SNP, where (G/C or C/C – “any C”) was hypothesized to have better outcome than G/G. The receptor D358A IL-6R SNP was grouped as C/C or A/C (“any C”), which correlates with lower sIL-6R serum levels26 (hypothesized “better” outcome), versus A/A (hypothesized “worse” outcome). The “better” outcome patients based on the -174 IL-6 (G>C) SNP were combined with the “better” outcome patients based on the D358A IL-6R SNP, and the EFS and OS were compared to the “worse” outcome patients from each SNP. Cross tabulation of the -174 IL-6 (G>C) SNP versus the D358A IL-6R SNP was performed.
Results
IL-6 SNP genotypes in neuroblastoma patients
The characteristics of high risk neuroblastoma patient sample set compared to known risk factors for high-risk disease are shown on Table 1. To determine the genotype of the two SNP known to have the greatest impact on serum levels of IL-6 (-174 IL-6 (G>C); rs1800795) and the soluble IL-6 receptor (D358A IL-6R; rs8192284), RFLP analysis was performed on 4 neuroblastoma tumor cell lines and 96 DNA samples from high risk neuroblastoma patients (Figure 1). Each RFLP analysis included an internal positive control to verify endonuclease activity, which gave rise to products of 75bp for the -174 IL-6 (G>C) SNP and 111bp for the D358A IL-6R SNP (Figure 1). While the allele frequencies of the D358A sIL-6R SNP were similar to those reported previously 27, the frequency of the -174 IL-6 (G>C) SNP was skewed by 54% toward the G/G genotype relative to published population genotypes 28–30 (Table 2). The proportion of patients in this high risk neuroblastoma cohort with the G/G genotype (57.3%) was statistically higher (P-value = 0.0003) than that of the general Caucasian population (37.1%, i.e., 299 of 807 28–30). Caucasians are highly polymorphic at the -174 IL-6 (G>C) SNP while African-Americans and Asians are predominantly G/G at this locus 28–30. Therefore, we examined whether the observed shift toward G/G at the -174 IL-6 (G>C) SNP in high risk neuroblastoma patients was due to the racial makeup of our random sample. Of the 96 samples that SNP data was obtained, 62.5%, 19.8%, 5.2%, and 12.5% were classified as White/Caucasian, Black/African-American, Asian/South-East Islander, and unknown/other, respectively. A similar skewing toward the G/G genotype at the -174 IL-6 (G>C) SNP was observed for the Caucasian subset (G/G = 53.3%, G/C = 38.3%, C/C = 8.3%), suggesting that differences in racial polymorphic frequencies did not influence the observed shift from previous population studies.
Table 1.
Characteristics of high risk neuroblastoma patient sample set (n = 96) compared to known risk factors.
| Factor | N (%) | -174 IL-6 (G>C) | |||
|---|---|---|---|---|---|
| (G/G) | (G/C) | (C/C) | Unknown | ||
| Age | |||||
| < 365 days | 12 (12%) | 7 | 4 | 1 | 0 |
| 365–547 days | 7 (7%) | 5 | 1 | 0 | 1 |
| ≥ 548 days | 79 (81%) | 43 | 28 | 7 | 1 |
| Sex | |||||
| Male | 58 (59%) | 35 | 18 | 4 | 1 |
| Female | 40 (41%) | 20 | 15 | 4 | 1 |
| INSS Stage (*all patient samples were COG High Risk) | |||||
| Stage 1 | 0 (0%) | 0 | 0 | 0 | 0 |
| Stage 2A/2B | 1 (1%) | 1 | 0 | 0 | 0 |
| Stage 3 | 17 (17%) | 8 | 7 | 2 | 0 |
| Stage 4 | 79 (81%) | 46 | 25 | 6 | 2 |
| Stage 4s | 1 (1%) | 0 | 1 | 0 | 0 |
| MYCN amplification | |||||
| Amplified | 40 (43%) | 25 | 13 | 2 | 0 |
| Not amplified | 53 (57%) | 26 | 19 | 6 | 2 |
| Unknown | 5 | 4 | 1 | 0 | 0 |
| Histology (Shimada) | |||||
| Favorable | 6 (8%) | 3 | 1 | 1 | 1 |
| Unfavorable | 74 (92%) | 40 | 27 | 6 | 1 |
| Unknown | 18 | 12 | 5 | 1 | 0 |
| 11q LOH | |||||
| Present | 26 (43%) | 15 | 8 | 3 | 0 |
| Absent | 34 (57%) | 16 | 13 | 3 | 2 |
| Unknown | 38 | 24 | 12 | 2 | 0 |
| 1p deletion | |||||
| Present | 27 (44%) | 14 | 10 | 3 | 0 |
| Absent | 34 (56%) | 19 | 10 | 4 | 1 |
| Unknown | 37 | 22 | 13 | 1 | 1 |
Table 2.
Allele frequencies for rs1800795 (IL-6 promoter) and rs8192284 (IL-6 receptor) SNP in high risk neuroblastoma (NBL) patients. Allele frequencies of two SNP known to impact IL-6 signaling events were compared between high risk NBL patients and those published in other population-based studies. While similar frequencies were noted between the IL-6 receptor SNP (rs8192284), a statistically significant shift toward G/G was observed the promoter SNP (rs1800795) in high risk NBL patients compared to the general population (P-value = 0.0003; see text for more details).
| Allele Frequencies | ||
|---|---|---|
| Genotype | High Risk Neuroblastoma Samples (%) | Population (%) (ref: *28–30; †27) |
| -174 IL-6 (G>C) (rs1800795) | ||
| G/G | 55 (57.3) | *37.1 |
| G/C | 33 (34.4) | *45.2 |
| C/C | 8 (8.3) | *17.7 |
| D358A IL6R (rs8192284) | ||
| A/A | 41 (42.7) | †34.5 |
| A/C | 52 (54.2) | †49.7 |
| C/C | 3 (3.1) | †15.8 |
Impact of IL-6 SNP biomarkers on EFS and OS in neuroblastoma
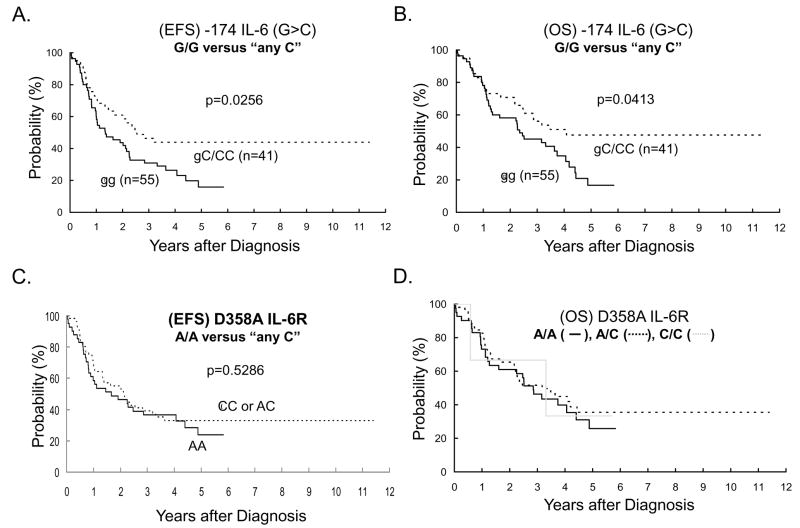
To determine if the -174 IL-6 (G>C) SNP and the D358A IL-6R SNP were predictive of neuroblastoma disease progression and mortality, we examined 3-year EFS and OS rates within the 96 genotyped DNA patient samples. Pairwise statistical analysis was completed for each of the three genotypes within both IL-6-related SNP, and further secondary comparisons were performed across each of the two SNP genotypes. Only the -174 IL-6 (G>C) SNP demonstrated statistically significant differences (P-value < 0.05) in predicting EFS and OS rates (Figure 2A and 2B). Patients carrying one or more C alleles had a higher OS than those who are homozygous for the G allele (3-year OS: 56% ± 8% versus 45% ± 7%; p=0.0413). Secondary analysis of EFS and OS curves of “better” versus “worse” outcome between the -174 IL6 (G>C) and D358A IL-6R SNP revealed no statistically significant differences (data not shown), which was not surprising since the D358A IL-6R polymorphisms were themselves not statistically significantly different (Figure 2C and 2D). Next, we determined whether the -174 IL-6 (G>C) SNP represented a novel, independent genetic biomarker for neuroblastoma. Current risk stratification for neuroblastoma includes age, INSS stage, MYCN status, INPC Histology, and DNA ploidy 1. In addition, 11q LOH, and 1p deletion are established adverse cytogenetic markers in neuroblastoma 1, 31. No statistically significant associations were found between the -174 IL-6 (G>C) SNP and any of these neuroblastoma risk stratification factors (Table 3). Interestingly, neuroblastoma patients with at least one C allele (i.e., any C) at the -174 IL-6 (G>C) SNP exhibited significantly better EFS and OS rates (Figure 2A and 2B). The -174 IL-6 (G>C) SNP remained independently prognostic after adjustment for MYCN amplification (Model B) or after adjustment for diploidy (Model C) (Table 3).
Figure 2.
Kaplan-Meier curves for event-free (EFS) and overall survival (OS) rates. EFS and OS rates were compared between G/G and “any C” (G/C or C/C) for the IL-6 promoter (rs1800795) SNP and between A/A and “any C” (A/C or C/C) for the IL-6 receptor (rs8192284) SNP.
Table 3.
Test for independent statistical significance of -174 IL6 SNP after adjustment for NB risk factors
| Factor | N (%) | -174 IL-6 (G>C) | |||
|---|---|---|---|---|---|
| (G/G) | (G/C) | (C/C) | Unknown | ||
| Age | |||||
| < 365 days | 12 (12%) | 7 | 4 | 1 | 0 |
| 365–547 days | 7 (7%) | 5 | 1 | 0 | 1 |
| ≥ 548 days | 79 (81%) | 43 | 28 | 7 | 1 |
| Sex | |||||
| Male | 58 (59%) | 35 | 18 | 4 | 1 |
| Female | 40 (41%) | 20 | 15 | 4 | 1 |
| INSS Stage (*all patient samples were COG High Risk) | |||||
| Stage 1 | 0 (0%) | 0 | 0 | 0 | 0 |
| Stage 2A/2B | 1 (1%) | 1 | 0 | 0 | 0 |
| Stage 3 | 17 (17%) | 8 | 7 | 2 | 0 |
| Stage 4 | 79 (81%) | 46 | 25 | 6 | 2 |
| Stage 4s | 1 (1%) | 0 | 1 | 0 | 0 |
| MYCN amplification | |||||
| Amplified | 40 (43%) | 25 | 13 | 2 | 0 |
| Not amplified | 53 (57%) | 26 | 19 | 6 | 2 |
| Unknown | 5 | 4 | 1 | 0 | 0 |
| Histology (Shimada) | |||||
| Favorable | 6 (8%) | 3 | 1 | 1 | 1 |
| Unfavorable | 74 (92%) | 40 | 27 | 6 | 1 |
| Unknown | 18 | 12 | 5 | 1 | 0 |
| 11q LOH | |||||
| Present | 26 (43%) | 15 | 8 | 3 | 0 |
| Absent | 34 (57%) | 16 | 13 | 3 | 2 |
| Unknown | 38 | 24 | 12 | 2 | 0 |
| 1p deletion | |||||
| Present | 27 (44%) | 14 | 10 | 3 | 0 |
| Absent | 34 (56%) | 19 | 10 | 4 | 1 |
| Unknown | 37 | 22 | 13 | 1 | 1 |
Discussion
We have demonstrated that the -174 IL-6 (G>C) SNP is an independent prognostic marker for clinical outcome in high risk neuroblastoma patients (Table 3 and Figure 2A and 2B). In contrast to the -174 IL-6 (G>C) SNP located in the IL-6 promoter, the D358A IL-6R receptor SNP failed to demonstrate any association between high risk neuroblastoma and disease progression or survival (Figure 2C and 2D).
In the Caucasian population, the published distribution of G/G, G/C and C/C polymorphisms at the -174 IL-6 (G>C) SNP promoter region is 37.1%, 45.6% and 17.3%, respectively 26–29. Individuals who harbor the homozygous G/G polymorphism produce IL-6 concentrations of [5.35 ± 3.01 pg/L] compared with those carrying G/C [3.96 ± 2.71 pg/ml] or C/C [3.52 ± 2.4 pg/ml] (G/G versus C/C, P-value < 0.05) 29 and other studies support a similar association between the G allele and elevated serum IL-6 levels in healthy volunteers 23, 24. Data from several studies have demonstrated that the -174 IL-6 (G>C) SNP results in a functional alteration which affects gene transcription and subsequent serum levels of IL-6 cytokine 23, 24, 26–28, 32–35. These data support the use of the -174 IL-6 (G>C) SNP genotype as a surrogate marker of cumulative IL-6 exposure. In our population of high risk neuroblastoma patients, 57.3% were carriers of the G/G polymorphism (Table 1), and the IL-6 promoter polymorphism identified two subsets of high risk neuroblastoma patients (Figure 2). In the first subset, individuals carrying one or more C alleles had a 3-year overall survival of 56% ± 8%, while those who are homozygous for the G allele had a lower overall survival, 45% ± 7% at 3-years (Figure 2B). As shown recently by Egler et al 14, patients with elevated IL-6 have significantly decreased EFS. The consequent elevated production of IL-6 in individuals with G/G SNP in the IL6 promoter region may be a plausible explanation for these data, suggesting that IL-6 may be a factor involved in neuroblastoma disease progression. Although elevated IL-6 level may be the consequence of advanced disease, our data suggest that the genetic makeup of the individual could also play a role in neuroblastoma disease progression. The correlation between IL-6 level, advanced disease and poor outcome may be part of a neuroblastoma-stimulated proliferation loop, similar to the one seen in multiple myeloma. Interestingly, stable physiological differences in the soluble IL-6 receptor due to the D358A sIL-6R polymorphism (e.g., A/A = 23.8 ng/ml, A/C = 29.7 ng/ml, C/C = 39.7 ng/[ml]26), did not impact clinical outcomes within the same patient population suggesting that elevated sIL6-R levels are not compensatory during limited bioavailability of IL-6 (Figure 2).
Neuroblastoma is a biologically heterogeneous tumor, with a spectrum of clinical presentations and responsiveness to therapy. Despite the ability to identify high-risk disease by numerous adverse prognostic biomarkers for neuroblastoma, the overall survival of high risk patients remains poor 1. The identification of yet another biomarker of inferior outcome in high-risk neuroblastoma in itself adds little. However, there is growing evidence that suggests that IL-6 may plays a role in neuroblastoma growth and dissemination. Retinoic acid has been shown to down modulate the expression of IL-6 receptor α chain 36, 37, inhibit secretion of IL-6 by stromal cells 37, and disrupt the IL-6 autocrine signaling pathway 38, 39. In CCG3891, it was demonstrated that the addition of cis-retinoic acid during the post-transplant consolidation phase of therapy was beneficial with improved EFS in high risk neuroblastoma patients 40. It is felt the primary benefit of cis-retinoic acid is inducing terminal differentiation in neuroblastoma cells; however, the role of cis-retinoic acid may also function by down regulating IL-6 signaling events, a hypothesis worthy of further investigation. Additionally, IL-6 can now be targeted therapeutically anti-IL-6 monoclonal antibodies which are commercially available (e.g., CNTO 328, Tocilizumab®).
Additional retrospective and prospective studies are underway to expand on our current data and to elucidate the functional consequences of the -174 IL-6 (G>C) promoter polymorphism within the entire neuroblastoma patient population. Data from this study adds to the growing literature of the association between the -174 IL-6 (G>C) SNP and clinical outcomes of various cancers. 11, 12, 20, 21
Supplementary Material
Acknowledgments
Financial support: Nationwide Children’s Hospital, Center for Childhood Cancer
We would like thank the high risk neuroblastoma patients and their parents for consenting to provide tissue samples. We would also like to thank the Children’s Oncology Group (COG) for supplying 96 DNA samples and the associated annotated clinical data.
Footnotes
Translational relevance
Recent studies suggest a link between interleukin-6 and more aggressive behavior in neuroblastoma tumor cells. The following study describes the distribution of IL-6 promoter and soluble IL-6 receptor single nucleotide polymorphism (SNP) genotypes in 96 high risk neuroblastoma patients. The study finds that rs1800795 IL-6 promoter SNP is a novel and independent prognostic factor for outcome in high risk neuroblastoma patients. The clinical relevance of these findings is two-fold. First, the finding that a germ-line SNP in the IL-6 promoter region is associated with neuroblastoma disease progression and survival provides a mechanistic link between host environment and tumor growth potential. Second, since rs1800795 SNP (-174 IL-6 (G>C) has been shown to correlate with IL-6 production, this cytokine may represent a target for development of new therapies in neuroblastoma.
References
- 1.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369(9579):2106–20. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.DuBois SG, Kalika Y, Lukens JN, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21(3):181–9. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer cell. 2008;13(1):7–9. doi: 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Weiss G, Goodnough LT. Anemia of chronic disease. The New England journal of medicine. 2005;352(10):1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 5.Voorhees PM, Chen Q, Kuhn DJ, et al. Inhibition of interleukin-6 signaling with CNTO 328 enhances the activity of bortezomib in preclinical models of multiple myeloma. Clin Cancer Res. 2007;13(21):6469–78. doi: 10.1158/1078-0432.CCR-07-1293. [DOI] [PubMed] [Google Scholar]
- 6.Becker C, Fantini MC, Schramm C, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21(4):491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Cardillo MR, Ippoliti F. Interleukin-6, interleukin-10 and heat shock protein-90 expression in renal epithelial neoplasias and surrounding normal-appearing renal parenchyma. International journal of immunopathology and pharmacology. 2007;20(1):37–46. doi: 10.1177/039463200702000105. [DOI] [PubMed] [Google Scholar]
- 8.Tesch H, Jucker M, Klein S, et al. Hodgkin and Reed-Sternberg cells express interleukin 6 and interleukin 6 receptors. Leukemia & lymphoma. 1992;7(4):297–303. doi: 10.3109/10428199209049781. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen LM, Klausen TW, Davidsen UH, et al. Early changes in serum IL-6 and VEGF levels predict clinical outcome following first-line therapy in aggressive non-Hodgkin’s lymphoma. Ann Hematol. 2005;84:510–516. doi: 10.1007/s00277-005-1020-x. [DOI] [PubMed] [Google Scholar]
- 10.George DJ, Halabi S, Shepard TF, et al. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: results from cancer and leukemia group B 9480. Clin Cancer Res. 2005;11:1815–20. doi: 10.1158/1078-0432.CCR-04-1560. [DOI] [PubMed] [Google Scholar]
- 11.Mouwad R, Rixe O, Meric JB, et al. Serum interleukin-6 concentrations as predictive factor of time to progression in metastatic malignant melanoma patients treated by biochemotherapy: a retrospective study. Cytokines Cell Mol Ther. 2002;7:151. doi: 10.1080/13684730210002328. [DOI] [PubMed] [Google Scholar]
- 12.DeMichele A, Martin AM, Mick R, et al. Interleukin-6 -174G-->C polymorphism is associated with improved outcome in high-risk breast cancer. Cancer research. 2003;63(22):8051–6. [PubMed] [Google Scholar]
- 13.Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. Faseb J. 2007;21(13):3763–70. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 14.Egler RA, Burlingame SM, Nuchtern JG, et al. Interleukin-6 and soluble interleukin-6 receptor levels as markers of disease extent and prognosis in neuroblastoma. Clin Cancer Res. 2008;14:7028–34. doi: 10.1158/1078-0432.CCR-07-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ara T, DeClerck YA. Mechanisms of invasion and metastasis in human neuroblastoma. Cancer metastasis reviews. 2006;25(4):645–57. doi: 10.1007/s10555-006-9028-9. [DOI] [PubMed] [Google Scholar]
- 16.Fukaya Y, Shimada H, Wang LC, Zandi E, DeClerck YA. Identification of galectin-3-binding protein as a factor secreted by tumor cells that stimulates interleukin-6 expression in the bone marrow stroma. The Journal of biological chemistry. 2008;283(27):18573–81. doi: 10.1074/jbc.M803115200. [DOI] [PubMed] [Google Scholar]
- 17.Sohara Y, Shimada H, DeClerck YA. Mechanisms of bone invasion and metastasis in human neuroblastoma. Cancer letters. 2005;228(1–2):203–9. doi: 10.1016/j.canlet.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 18.Candi E, Knight RA, Spinedi A, Guerrieri P, Melino G. A possible growth factor role of IL-6 in neuroectodermal tumours. Journal of neuro-oncology. 1997;31(1–2):115–22. doi: 10.1023/a:1005706019048. [DOI] [PubMed] [Google Scholar]
- 19.Schumann G, Huell M, Machein U, Hocke G, Fiebich BL. Interleukin-6 activates signal transducer and activator of transcription and mitogen-activated protein kinase signal transduction pathways and induces de novo protein synthesis in human neuronal cells. Journal of neurochemistry. 1999;73(5):2009–17. [PubMed] [Google Scholar]
- 20.Kominsky SL, Davidson NE. A “bone” fide predictor of metastasis? Predicting breast cancer metastasis to bone. J Clin Oncol. 2006;24(15):2227–9. doi: 10.1200/JCO.2005.05.5319. [DOI] [PubMed] [Google Scholar]
- 21.Sasser AK, Mundy BL, Smith KM, et al. Human bone marrow stromal cells enhance breast cancer cell growth rates in a cell line-dependent manner when evaluated in 3D tumor environments. Cancer letters. 2007;254(2):255–64. doi: 10.1016/j.canlet.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Bennermo M, Held C, Stemme S, et al. Genetic predisposition of the interleukin-6 response to inflammation: implications for a variety of major diseases? Clinical chemistry. 2004;50(11):2136–40. doi: 10.1373/clinchem.2004.037531. [DOI] [PubMed] [Google Scholar]
- 23.Rivera-Chavez FA, Peters-Hybki DL, Barber RC, O’Keefe GE. Interleukin-6 promoter haplotypes and interleukin-6 cytokine responses. Shock (Augusta, Ga) 2003;20(3):218–23. doi: 10.1097/01.shk.0000079425.52617.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galicia JC, Tai H, Komatsu Y, Shimada Y, Akazawa K, Yoshie H. Polymorphisms in the IL-6 receptor (IL-6R) gene: strong evidence that serum levels of soluble IL-6R are genetically influenced. Genes and immunity. 2004;5(6):513–6. doi: 10.1038/sj.gene.6364120. [DOI] [PubMed] [Google Scholar]
- 25.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J Royal Stat Soc Series A. 1972;135:185–198. [Google Scholar]
- 26.Reich D, Patterson N, Ramesh V, et al. Admixture mapping of an allele affecting interleukin 6 soluble receptor and interleukin 6 levels. American journal of human genetics. 2007;80(4):716–26. doi: 10.1086/513206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. The Journal of clinical investigation. 1998;102(7):1369–76. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Packer BR, Yeager M, Burdett L, et al. SNP500Cancer: a public resource for sequence validation, assay development, and frequency analysis for genetic variation in candidate genes. Nucleic acids research. 2006;34(Database issue):D617–21. doi: 10.1093/nar/gkj151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walston J, Arking DE, Fallin D, et al. IL-6 gene variation is not associated with increased serum levels of IL-6, muscle, weakness, or frailty in older women. Experimental gerontology. 2005;40(4):344–52. doi: 10.1016/j.exger.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Attiyeh EF, London WB, Mosse YP, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. The New England journal of medicine. 2005;353(21):2243–53. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 31.Hulkkonen J, Pertovaara M, Antonen J, Pasternack A, Hurme M. Elevated interleukin-6 plasma levels are regulated by the promoter region polymorphism of the IL6 gene in primary Sjogren’s syndrome and correlate with the clinical manifestations of the disease. Rheumatology (Oxford, England) 2001;40(6):656–61. doi: 10.1093/rheumatology/40.6.656. [DOI] [PubMed] [Google Scholar]
- 32.Belluco C, Olivieri F, Bonafe M, et al. -174 G>C polymorphism of interleukin 6 gene promoter affects interleukin 6 serum level in patients with colorectal cancer. Clin Cancer Res. 2003;9(6):2173–6. [PubMed] [Google Scholar]
- 33.Castell JV, Gomez-Lechon MJ, David M, et al. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS letters. 1989;242(2):237–9. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- 34.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. The Journal of biological chemistry. 2000;275(24):18138–44. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 35.Granchi D, Garaventa A, Amato I, Paolucci P, Baldini N. Plasma levels of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in patients with neuroblastoma. International journal of cancer. 2006;119(1):146–51. doi: 10.1002/ijc.21783. [DOI] [PubMed] [Google Scholar]
- 36.Kawano M, Tanaka H, Ishikawa H, et al. Interleukin-1 accelerates autocrine growth of myeloma cells through interleukin-6 in human myeloma. Blood. 1989;73(8):2145–8. [PubMed] [Google Scholar]
- 37.Sidell N, Taga T, Hirano T, Kishimoto T, Saxon A. Retinoic acid-induced growth inhibition of a human myeloma cell line via down-regulation of IL-6 receptors. J Immunol. 1991;146(11):3809–14. [PubMed] [Google Scholar]
- 38.Ogata A, Nishimoto N, Shima Y, Yoshizaki K, Kishimoto T. Inhibitory effect of all-trans retinoic acid on the growth of freshly isolated myeloma cells via interference with interleukin-6 signal transduction. Blood. 1994;84(9):3040–6. [PubMed] [Google Scholar]
- 39.Chen YH, Desai P, Shiao RT, Lavelle D, Haleem A, Chen J. Inhibition of myeloma cell growth by dexamethasone and all-trans retinoic acid: synergy through modulation of interleukin-6 autocrine loop at multiple sites. Blood. 1996;87(1):314–23. [PubMed] [Google Scholar]
- 40.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. The New England journal of medicine. 1999;341(16):1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.