Figure 8. Start-trap experiments using ASF/SF2 with modified RS domains.
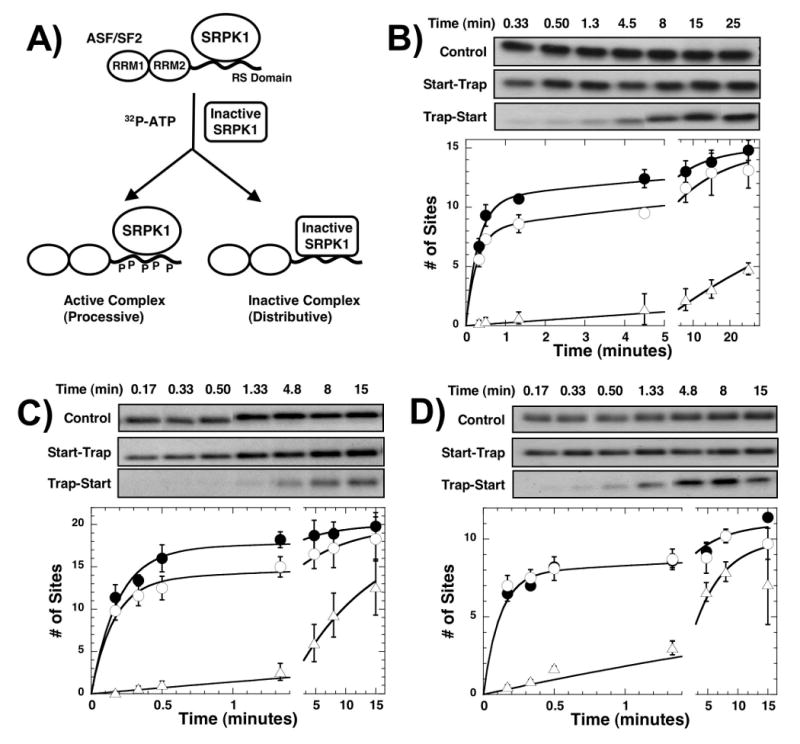
A) Start-trap protocol. (B-D) Start-trap experiments. SRPK1 (1μM) is preincubated with 200 nM of wt-ASF/SF2 (B), RS8-RS8 (C), and SA8-RS8 (D) and then the reaction is started with 100 μM 32P-ATP in the absence (control, ●) and presence of 30 μM kdSRPK1 (start-trap, ○). In trap-start experiments, the complex is first mixed with kdSRPK1 before 32P-ATP is added (△). The experiments are performed in triplicate and error bars for each time point are displayed. One representative kinetic experiment for each substrate is shown in the gel panels. The amplitudes of the fast phases are 11 ± 0.60 and 8.0 ± 0.36 sites in the absence and presence of kdSRPK1 for wild-type ASF/SF2, 17 ± 1.0 and 14 ± 0.80 sites in the absence and presence of kdSRPK1 for RS8-RS8, and 7.8 ± 0.56 and 7.8 ± 0.48 sites in the absence and presence of kdSRPK1 for SA8-RS8.
