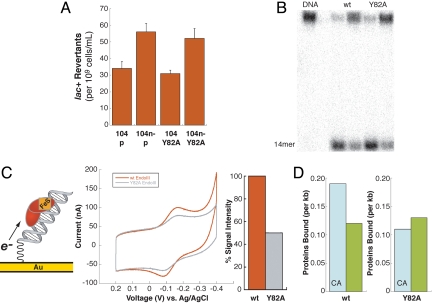
Fig. 4.
Y82A EndoIII, a mutant in DNA-mediated CT capability. (A) Bar graph showing lac+ revertants for CC104/p, CC104 nth−/p, CC104/Y82A, and CC104 nth−/Y82A strains, where p denotes inclusion of an empty vector. Lac+ revertants are reported as the average number of lac+ colonies that arise per 109 cells plated on minimal lactose medium containing ampicillin. Data for the CC104 strains are shown based on five sets of independent experiments, each containing 10 replicates per strain. (B) Autoradiogram after denaturing PAGE of 32P-5′-TGTCAATAGCAAGXGGAGAAGTCAATCGTGAGTCT-3′ plus complementary strand, where X = 5-OH-dU base-paired with G. Protein samples (100 or 10 nM) were incubated with duplexes for 15 min at 37 °C and quenched with 1 M NaOH. Cleavage of the 32P-labeled strand at the lesion site (X) by EndoIII results in formation of a 14-mer. No significant difference in glycosylase activity (10% uncertainty) is observed between Y82A and WT EndoIII. (C) Cyclic voltammetry of Y82A EndoIII at an Au electrode modified with SH(CH2)2CONH(CH2)6NHOCO-5′-AGTACAGTCATCGCG-3′ plus complementary strand showing the reduction and reoxidation of the DNA-bound protein. DNA-modified surfaces were prepared and backfilled with mercaptohexanol, and WT or Y82A EndoIII was tested. Surfaces were then rinsed, and the other protein was analyzed on the same surface. Over several trials, the electrochemical signal associated with Y82A was 50% ± 13% smaller per [4Fe4S] cluster compared with WT EndoIII, reflecting poor electronic coupling of the mutant to the DNA-modified electrode. (D) Comparative densities for WT (Left) and Y82A (Right) EndoIII bound to matched versus mismatched (CA) strands measured by AFM. Although WT EndoIII preferentially redistributes onto the mismatched strand, Y82A shows no preference.