Abstract
Ammonoids and conodonts, being characterized by exceptionally high background rates of origination and extinction, were vulnerable to global environmental crises, which characteristically intensified background rates of extinction. Thus, it is not surprising that these taxa suffered conspicuous mass extinctions at the times of three negative Early Triassic global carbon isotopic excursions that resembled those associated with the two preceding Permian mass extinctions. In keeping with their high rates of origination, both the ammonoids and conodonts rediversified dramatically between the Early Triassic crises. Other marine taxa, characterized by much lower intrinsic rates of origination, were held at low levels of diversity by the Early Triassic crises; because global mass extinctions affect all marine life, these taxa must have experienced relatively modest expansions and contractions that have yet to be discovered, because they do not stand out in the fossil record and because the stratigraphic ranges of these taxa, being of little value for temporal correlation, have not been thoroughly studied.
Keywords: carbon isotopes, Permian
The greatest mass extinction of the past half billion years occurred at the end of the Permian Period. A substantial biotic recovery was delayed until the end of the Early Triassic Epoch, which lasted ≈6 million years (My). After the destruction of coniferous vegetation, lycopod spore plants came to dominate the land (1), and total benthic marine diversity remained relatively low until the Middle Triassic time (2). This was an unusually long delay for biotic recovery after a mass extinction (3), and the Early Triassic has therefore often been viewed as an interval when global conditions remained hostile to life (4–6). Both the terminal Permian crisis and a lesser mass extinction at the end of the Middle Permian were accompanied by a sharp negative shift of stable carbon isotopes, as recorded in limestones and organic carbon in numerous marine and terrestrial strata (reviewed by Retallack et al., ref. 7). Three similar negative isotope spikes have been found in Early Triassic marine carbonate rocks (8). Similar excursions in paleosols (9) may correlate with them. In contrast, the carbon isotope curve for the Middle Triassic is quite stable after an initial positive shift (Fig. 1). The discovery of negative excursions in the Griesbachian, Smithian, and Spathian stages of the Early Triassic has led to the hypothesis that a succession of ecological crises similar to those of the Permian stifled recovery from the Permian crisis (9–12). Thus, intervals between crises might have been quite hospitable to life but too brief to permit substantial diversification by most taxa. The present paper confirms this hypothesis.
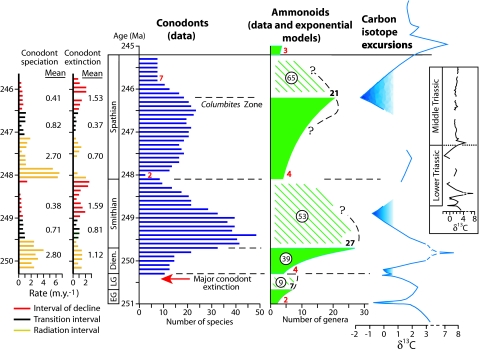
Fig. 1.
Simultaneous radiations and mass extinctions of conodonts and ammonoids in Early Triassic time, and negative carbon isotope excursions during intervals of mass extinction. Numbers of conodont species are three-interval moving averages for 100,000-year intervals based on a global compilation (19) from which rates of speciation and extinction were also calculated; uncertain range extensions were not included. Bold black numbers give standing global diversities of ammonoid genera at the end of the Griesbachian and Dienerian (14–16) and the top of the Columbites Zone (42). Encircled numbers give total diversity for the post-Columbites Zone interval (42) and for earlier intervals (14–16). For hatched intervals, the exact pattern of change for ammonoid diversity is uncertain; for solid green intervals, diversification is modeled as exponential, but for the Spathian, the rate may have begun to decline earlier than shown (see dashed line), paralleling the pattern for conodonts. In red are numbers of conodont species and ammonoid genera at ends of mass extinction intervals. The Early Triassic carbon isotope graph is simplified from a composite plot for data from four stratigraphic sections in South China (9, 25). It is rescaled to be compatible with the time scale from Gradstein and Ogg (43) used here. The curve for the Middle Triassic (Inset) represents a single section in South China (9).
Rates of evolutionary radiation and background extinction for taxa tend to be positively correlated because these rates are governed by the same biological traits; furthermore, taxa that have high background rates of extinction tend to suffer preferentially in biological crises, during which background rates are multiplied (13). Taxa characterized by very high rates of evolutionary radiation and extinction might therefore be expected to display conspicuous patterns of expansion and decline during the Early Triassic. The two such marine taxa with well-studied Lower Triassic fossil records are the ammonoids and conodonts, and an analysis of their history indicates that they suffered mass extinctions at the times of the negative carbon isotope shifts and rediversified markedly during the intervening intervals.
The extensive compilations of Tozer (14–16) are the basis for the following analysis. Tozer's assembly of Lower Triassic ammonoid occurrences (14–16), although somewhat dated, represents a large, global sample and has recently been used effectively to analyze patterns of ammonoid evolution after the terminal Permian mass extinction (17, 18). Orchard (19) has recently analyzed Lower Triassic conodont occurrences on a global scale with the remarkable resolution of ≈100,000 years.
Results
Not surprisingly, the patterns of diversification and extinction of Early Triassic ammonoids and conodonts turn out to be remarkably similar (Fig. 1). Orchard (19) noted that each extinction interval for the conodonts was associated with a pronounced global negative carbon isotope excursion. In fact, in each case, heavy extinction began before the minimum isotopic value was reached (Fig. 1). The ammonoid extinction events were also temporally associated with these isotopic excursions, but the precise timing relative to the excursions is less clear. After the terminal Permian crisis, both the ammonoids and the conodonts experienced rapid recovery during the Griesbachian Age, followed by a major extinction. Taxonomic and stratigraphic uncertainties prevent a rigorous assessment of what clearly was a strong pulse of conodont extinction near the end of the Griesbachian: very few species of the two most diverse conodont families of this interval (the Neogondolellinae and Anchignathodontidae) survived into the Dienerian (19). Then, both the ammonoids and conodonts radiated rampantly during Dienerian and early Smithian time, and both suffered another mass extinction during the Smithian. For the conodonts, the Smithian crisis spanned ≈1 My, with only two species known to have persisted into the Spathian; similarly, only four ammonoid genera are known to have survived into the Spathian (Fig. 1). The Spathian radiation was slower than the Dienerian radiation for both taxa. Another mass extinction for the conodonts in late Spathian time spanned ≈0.6 My. The Smithian and Spathian declines for the ammonoids were presumably also protracted because, like the conodonts, the ammonoids did not radiate persistently throughout either interval: 27 ammonoid genera are known to have survived into the Smithian, yet a total of only 53 genera are recognized for this entire interval, and the corresponding numbers for the interval above the Columbites zone of the Spathian are 21 and 65 (Fig. 1).
The reduction of ammonoid and conodont diversity to very low levels at the end of the Griesbachian, Smithian, and Spathian and the subsequent appearance of new subtaxa must represent the kinds of biotic changes that long ago led to the recognition of these intervals as formal stages. The absence of heavy extinction late in the Dienerian explains the historical grouping of this interval with the Smithian as the Nammalian Stage.
The Spathian decline for the ammonoids has been recognized previously (11), as have all three Early Triassic declines for the conodonts (19). It seems never to have been generally recognized, however, that three major extinctions, entailing both taxa, occurred in the Early Triassic, and that these events must have been aspects of global mass extinctions for marine life in general.
In Fig. 1, the Dienerian–Spathian history of the conodonts is divided into intervals of radiation and decline and intervening intervals of transition. Significantly, the Smithian and Spathian intervals of mass extinction for conodonts both entailed not only increased rates of extinction but also depressed rates of speciation (Fig. 1). These were not intervals of high taxonomic turnover (19); in each case, a severe decline was followed by an interval of accelerated speciation.
Intervals of radiation for conodonts were characterized by remarkably high rates of speciation. Direct calculations from the standing diversities and stratigraphic ranges of species (19) give a mean rate of speciation (O) during the Dienerian/Smithian conodont radiation of 2.80 My−1 and a mean rate of extinction (E) of 1.12 My−1. This implies a net rate of exponential increase for this group of nektonic marine vertebrates (R = O − E) of 1.68 My−1, which is much higher than that for nearly all marine invertebrates (13); the doubling time (t2) of 0.41 My for conodont radiation was remarkably short.
Like the conodonts, the ammonoids diversified more rapidly and attained higher diversity during their radiation that began in the Dienerian than during their Spathian radiation (Fig. 1). Possibly, global environmental conditions damped the radiations of both taxa in Spathian time. Because stratigraphic ranges of taxa are less well constrained for ammonoids than for conodonts, it is necessary to assess the ammonoids' Early Triassic diversity changes at the genus level and to use indirect methods to estimate rates of change. The following equation gives the rate of increase (R) for the Dienerian ammonoid radiation, assuming that it followed the approximately exponential pattern that typifies marine radiations (13, 20):
where N0 is the original number of genera, N is the final number, t is the duration of the radiation, and e is the base for natural logarithms.
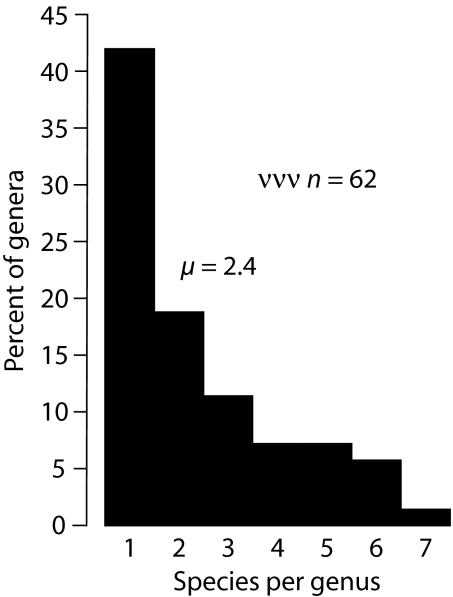
For the Dienerian radiation, R = 3.18 (t2 = 0.22 My). The rate at the species level would have been the same as this if a particular species-to-genus ratio held throughout the Dienerian radiation. The two rates would have been similar even if the species-to-genus ratio varied somewhat, because an average Early Triassic ammonoid genus contained only ≈2.4 known species, and ≈42% of recognized genera are monospecific (Fig. 2). For example, if all four genera present at the start of the Dienerian had been monospecific and the 27 genera at the end contained 65 species (an average of 2.4 per genus), then R for species would have been 4.65 My−1 (t2 = 0.15 My), which differs only modestly from the generic rate.
Fig. 2.
Frequency distribution of numbers of species per genus for ammonoids of the post-Columbites interval of Early Triassic time (42).
For the Dienerian ammonoid radiation, given empirical values of R, N, and Ñ (the total number of genera produced) (Fig. 1), one can calculate O and E as follows (derivation provided by Owen M. Phillips, Johns Hopkins University, Baltimore, MD) (13, 21). If N genera were present in the midst of this adaptive radiation, the number of genera Nt at some previous time T can be obtained by first calculating exponential decrease from N at the rate (R) at which the increase occurred:
where t is negative, equaling −T. The number of genera that died out in the next interval of time will equal the extinction rate, E, times the number of lineages present:
The total number of genera terminated since the start of a monophyletic radiation is then the summation for all past intervals:
The total number of genera that the radiation produced is this number plus N, the number extant at the end of the radiation:
which yields E, and therefore O, because O = R + E.
The above formulation assumes monophyly, but the Dienerian radiation began with four genera (Fig. 1). Therefore, for the calculation, N (which equals 27) must be reduced to 6.75, and Ñ (which equals 39) reduced to 9.8. The values of Ñ, N, and R for the ammonoids' radiation give E = 1.44 My−1 and O = 4.62 My−1. Mean duration of genera (D = E−1) during the radiation, therefore, was 0.70 My. This result compares favorably with the estimate of ≈0.5 My for average species duration of Jurassic and Cretaceous ammonoids (22) because, as noted above, ≈42% of Early Triassic ammonoid genera contained only one recognized species (Fig. 2). Furthermore, “average” in Kennedy (22) is likely to have referred to modal or median, rather than mean, duration.
Rates noted above for conodont species extant during the Dienerian/Smithian radiation (O = 2.80 My−1 and E = 1.12 My−1) are of the same order of magnitude as rates calculated for ammonoid genera, with the somewhat higher O for ammonoids resulting in a higher R. Mean duration of conodont species is similar to that of ammonoid species and genera: 0.90 My for the Dienerian/Smithian radiation (Fig. 1) and 0.80 My for the entire Dienerian-Smithian interval.
Discussion
All rates (O, E, and R) for Early Triassic ammonoids and conodonts were much higher than those that characterize most other marine taxa (R ≈ 0.06–0.4 My−1, E ≈ 0.04–0.33 My−1, S ≈ 0.1–0.74 My−1) (13). Bivalve and gastropod mollusks are relatively abundant taxa in Early Triassic rocks. For extant genera and families of these taxa that have arisen at some time within the past 100 My and diversified more or less exponentially (with allowance for the terminal Cretaceous mass extinction for taxa that arose before it occurred), the mean rate of radiation has been only 0.076 per 1 My (t2 = 9.1 My). Thus, values of R for ammonoids in the Early Triassic were perhaps 20–40 times higher than typical values for radiating taxa of bivalves and gastropods. Triassic diversities also indicate this kind of disparity: a total of 132 ammonoid genera are recorded for the Early Triassic (14–16), and the large majority of these descended from the single genus Xenodiscus at the start of the epoch (14, 15). In contrast, 32 bivalve genera and 49 gastropod genera are recorded for the start of the Early Triassic, yet the generic numbers for these groups for the entire interval are only 63 and 67, respectively; the equivalent numbers for articulate brachiopods are 12 and 33 (23).
Dramatic evolutionary recoveries by ammonoids and conodonts between the Early Triassic mass extinctions suggest that although the ocean became increasingly stratified during the Late Permian and remained in this state during the Early Triassic (24), marine taxa in general were not held back by persistently hostile environmental conditions. Rather, as hypothesized previously (9–12, 25), mass extinctions were so frequent that most taxa were unable to recover markedly until the end of the epoch, and their losses in the three mass extinctions were also too light to have stood out in the fossil record. This appears to be true for common Early Triassic benthic taxa, such as bivalves, gastropods, and brachiopods, all of which are characterized by much lower characteristics of rates of diversification and extinction than ammonoids and conodonts (23). Nonetheless, these taxa must have suffered modest losses in the three Early Triassic mass extinctions because all other marine mass extinctions have struck all taxa living in the ocean, albeit to differing degrees. The temporal ranges of the Early Triassic genera and species of bivalves, gastropods, and brachiopods have never been evaluated carefully because of their limited value for stratigraphic correlation; these ranges warrant detailed study.
Ecological changes in local faunas of the Early Triassic actually reflect modest recoveries by benthos between times of crisis. A substantial increase in species richness of faunas in well-oxygenated, shallow marine habitats during the Griesbachian time suggests that anoxia at greater depths has led to underestimation of the rediversification by benthos after the terminal Permian mass extinction (26, 27). Trace fossil faunas also indicate a substantial diversification of burrowing taxa during the Dienerian and Spathian (26, 28). In addition, gymnosperm floras reclaimed the land at the start of Spathian time (11).
Both the ammonoids and conodonts appear to have been mobile carnivores, and like most carnivores, they were probably selective in their diets. It is possible, however, that many ammonoids were microphagous (29). The modern genus Conus is a highly selective carnivorous gastropod, with many species focusing on a single species of prey (30). The rate of radiation of Conus during the late Cenozoic has been remarkably high compared with rates for gastropod grazers and bivalve suspension and deposit feeders, all of which are relatively unselective in their food consumption. Small populations of the latter taxa that constitute new species have little chance of expanding their populations and are likely to suffer rapid extinction because they have the same generalized feeding habits as their congeners. However, an incipient species of Conus, when focusing on a prey species unexploited by its congeners, is likely to undergo a population explosion and survive (31). It is reasonable to attribute, in large part, the very high rates of speciation of the predatory ammonoids and conodonts to this same kind of trophic specialization. Specialized reproductive behavior in these groups, readily leading to reproductive isolation via minor changes, may also have contributed to their high speciation rates; specialized behavior also rendered ammonoid and conodont species vulnerable to environmental change and, presumably, therefore contributed to their high rates of extinction (13, 32).
The factors most often hypothesized to have produced the two large negative isotope excursions of the Permian are: destruction of primary producers and oxidation of their organic carbon, which is isotopically light; a reduced rate of burial of organic carbon; and release of bacterially generated methane hydrates from the sea floor as a result of global warming (reviewed by Berner in ref. 33). Other suggested causes are a shift from algae and cyanobacteria to sulfur bacteria as the dominant photosynthesizers in the ocean (34) and release of large volumes of isotopically light carbon as the magmas that produced the massive Siberian Traps rose burned their way through the world's largest coal deposit (35). In fact, any of the five isotopic excursions considered here may have had more than one agent, perhaps as a result of positive feedback. Climatic warming at the time of the terminal Permian crisis (36) may have played a dominant role in the extinctions. Also hypothesized as agents are poisonous emissions of CO2 (37) or H2S (38) from the deep sea, extensive oceanic anoxia (39), and a sharp decline in atmospheric pO2 as atmospheric pCO2 rose (40, 41). Whatever disrupted the biosphere in the latter part of the Permian and early in the Triassic, the association of the repeated isotopic excursions with mass extinctions indicates that these excursions represent significant changes in the global carbon cycle. Explanation of these changes may shed light on the causes or consequences of the associated biotic crises.
Acknowledgments.
I thank Jonathan Payne and David Bottjer for helpful comments on the manuscript and Jennifer Engels for careful spreadsheet preparation and proofreading.
Footnotes
The author declares no conflict of interest.
References
- 1.Looy CV, Brugman WA, Dilcher DL, Visscher H. The delayed resurgence of equatorial forests after the Permian-Triassic ecologic crisis. Proc Natl Acad Sci USA. 1999;96:13857–13862. doi: 10.1073/pnas.96.24.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Twitchett RJ, Looy CV, Morante R, Visscher H, Wignall PB. Rapid and synchronous collapse of marine and terrestrial ecosystems during the end-Permian biotic crisis. Geology. 2001;29:351–354. [Google Scholar]
- 3.Erwin DH. The end and the beginning: Recoveries from mass extinctions. Trends Ecol Evol. 1998;13:344–349. doi: 10.1016/s0169-5347(98)01436-0. [DOI] [PubMed] [Google Scholar]
- 4.Woods AD, Bottjer DJ, Mutti M, Morrison J. Lower Triassic large sea-floor carbonate cements: Their origin and a mechanism for the prolonged biotic recovery from the end-Permian mass extinction. Geology. 1999;27:645–648. [Google Scholar]
- 5.Pruss S, Fraiser M, Bottjer DJ. Proliferation of Early Triassic wrinkle structures: Implications for environmental stress following the end-Permian mass extinction. Geology. 2004;32:461–464. [Google Scholar]
- 6.Peng Y, Shi GR, Gao Y, He W, Shen S. How and why did the Lingulidae (Brachiopoda) not only survive the end-Permian mass extinction but also thrive in its aftermath? Palaeogeogr Palaeoclimatol Palaeoecol. 2007;252:118–131. [Google Scholar]
- 7.Retallack GJ, et al. Middle-Late Permian mass extinction on land. Geol Soc Am Bull. 2006;118:1398–1411. [Google Scholar]
- 8.Payne JL, et al. Large perturbations of the carbon cycle during recovery from the end-Permian extinction. Science. 2004;305:506–509. doi: 10.1126/science.1097023. [DOI] [PubMed] [Google Scholar]
- 9.Krull ES, Retallack GJ. δ13C depth profiles from paleosols across the Permian-Triassic boundary: Evidence for methane release. GSA Bull. 2000;112:1459–1472. [Google Scholar]
- 10.Galfetti T, et al. Timing of the Early Triassic carbon cycle perturbations inferred from new U-Pb ages and ammonoid biochronozones. Earth Planet Sci Lett. 2007;258:593–604. [Google Scholar]
- 11.Galfetti T, et al. Smithian-Spathian boundary event: Evidence for global climatic change in the wake of the end-Permian biotic crisis. Geology. 2007;35:291–294. [Google Scholar]
- 12.Horacek M, Richoz S, Brandner R, Krystyn L, Spotl C. Evidence for recurrent changes in Lower Triassic oceanic circulation of the Tethys: The δ13C record from marine sections in Iran. Palaeogeogr Palaeoclimatol Palaeoecol. 2007;252:355–369. [Google Scholar]
- 13.Stanley SM. Macroevolution: Pattern and Process. New York: Freeman; 1979. [Google Scholar]
- 14.Tozer ET. Triassic ammonoids: Geographic and stratigraphic distribution. In: House MR, Senior JR, editors. The Ammonoidea. Vol 18. London: Academic; 1981. pp. 397–431. [Google Scholar]
- 15.Tozer ET. Triassic Ammonoidea: Classification, evolution and relationship with Permian and Jurassic forms. In: House MR, Senior JR, editors. The Ammonoidea. Vol 18. London: Academic; 1981. pp. 66–100. [Google Scholar]
- 16.Tozer ET. Canadian Triassic Ammonoid Faunas. Ottawa: Geological Survey of Canada; 1994. [Google Scholar]
- 17.McGowan AJ. Ammonoid recovery from the Late Permian mass extinction event. Comptes Rendus Palevol. 2005;4:517–530. [Google Scholar]
- 18.McGowan AJ. Ammonoid taxonomic and morphologic recovery patterns after the Permian-Triassic. Geology. 2004;32:665–668. [Google Scholar]
- 19.Orchard MJ. Conodont diversity and evolution through the latest Permian and Early Triassic upheavals. Palaeogeogr Palaeoclimatol Palaeoecol. 2007;252:93–117. [Google Scholar]
- 20.Stanley SM. A new analysis of the history of marine animal diversity. Paleobiology. 2007;33(Suppl):1–55. [Google Scholar]
- 21.Stanley SM. Trends, rates, and patterns of evolution in the Bivalvia. In: Hallam A, editor. Patterns of Evolution as Illustrated by the Fossil Record. Amsterdam: Elsevier; 1977. pp. 209–250. [Google Scholar]
- 22.Kennedy WJ. Ammonite evolution. In: Hallam A, editor. Patterns of Evolution as Illustrated by the Fossil Record. Amsterdam: Elsevier; 1977. pp. 251–304. [Google Scholar]
- 23.Sepkoski JJ. A compendium of fossil marine animal genera. Bull Am Paleontol. 2002:363. [Google Scholar]
- 24.Isozaki Y. Permo-Triassic boundary superanoxia and stratified superocean: Records from lost deep sea. Science. 1997;276:235–238. doi: 10.1126/science.276.5310.235. [DOI] [PubMed] [Google Scholar]
- 25.Payne JL, Kump LR. Evidence for recurrent Early Triassic massive volcanism from quantitative interpretation of isotope fluctuations. Earth Planet Sci Lett. 2007;256:264–277. [Google Scholar]
- 26.Twitchett RJ, Krystyn L, Baud A, Wheeley JR, Richoz S. Rapid marine recovery after the end-Permian mass-extinction event in the absence of marine anoxia. Geology. 2004;32:805–808. [Google Scholar]
- 27.Beatty TW, Zonneveld JP, Henderson CM. Anomalously diverse Early Triassic ichnofossil assemblages in northwest Pangea: A case for a shallow-marine habitable zone. Geology. 2008;36:771–774. [Google Scholar]
- 28.Twitchett RJ, Barras CG. Trace fossils in the aftermath of mass extinction events. In: Mcllroy D, editor. The Application of Ichnology to Palaeoenvironmental and Stratigraphic Analysis. Vol 228. London: Geol Soc London; 2004. p. 397.p. 418. [Google Scholar]
- 29.Fischer AG, Bottjer DJ. Oxygen-depleted waters: A lost biotope and its role in ammonite and bivalve evolution. Neues Jahrb Geol Palaontol Abh. 1995;195:133–146. [Google Scholar]
- 30.Kohn AJ. Tempo and mode of evolution in Conidae. Malacologia. 1990;32:55–67. [Google Scholar]
- 31.Stanley SM. Predation defeats competition on the seafloor. Paleobiology. 2008;34:1–21. [Google Scholar]
- 32.Stanley SM. The general correlation between rate of speciation and rate of extinction: Fortuitous causal linkages. In: Ross RM, Allmon WD, editors. Causes of Evolution. Chicago: Univ of Chicago Press; 1990. pp. 103–172. [Google Scholar]
- 33.Berner RA. Examination of hypotheses for the Permo-Triassic boundary extinction by carbon cycle modeling. Proc Natl Acad Sci USA. 2002;99:4172–4177. doi: 10.1073/pnas.032095199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riccardi A, Kump LR, Arthur MA, D'Hondt S. Carbon isotopic evidence for chemocline upward excursions during the end-Permian event. Palaeogeogr Palaeoclimatol Palaeoecol. 2007;248:73–81. [Google Scholar]
- 35.Erwin DH. Extinction: How Life on Earth Nearly Ended 250 Million Years Ago. Princeton: Princeton Univ Press; 2006. [Google Scholar]
- 36.Retallack GJ. Postapocalyptic greenhouse paleoclimate revealed by earliest Triassic paleosols in the Sydney Basin, Australia. Geol Soc Am Bull. 1999;111:52–70. [Google Scholar]
- 37.Knoll AH, Bambach RK, Canfield DE, Grotzinger JP. Comparative Earth history and Late Permian mass extinction. Science. 1996;273:452–457. [PubMed] [Google Scholar]
- 38.Kump LR, Pavlov A, Arthur MA. Massive release of hydrogen sulfide to the surface ocean and atmosphere during intervals of oceanic anoxia. Geology. 2005;33:397–400. [Google Scholar]
- 39.Wignall PB, Hallam A. Anoxia as a cause of the Permian/Triassic mass extinction; facies evidence from northern Italy and the western United States. Palaeogeogr Palaeoclimatol Palaeoecol. 1992;93:21–46. [Google Scholar]
- 40.Weidlich O, Kiessling W, Fluegel E. Permian-Triassic boundary interval as a model for forcing marine ecosystem collapse by long-term atmospheric oxygen drop. 2003;31:961–964. [Google Scholar]
- 41.Huey RB, Ward PD. Hypoxia, global warming, and terrestrial Late Permian extinctions. Science. 2005;308:398–401. doi: 10.1126/science.1108019. [DOI] [PubMed] [Google Scholar]
- 42.Kummel B. Ammonoids of the Late Scythian. Bull Mus Comp Zool. 1969;137:311–702. [Google Scholar]
- 43.Gradstein FM, Ogg JG. A Geologic Time Scale. Cambridge, UK: Cambridge Univ Press; 2004. [Google Scholar]