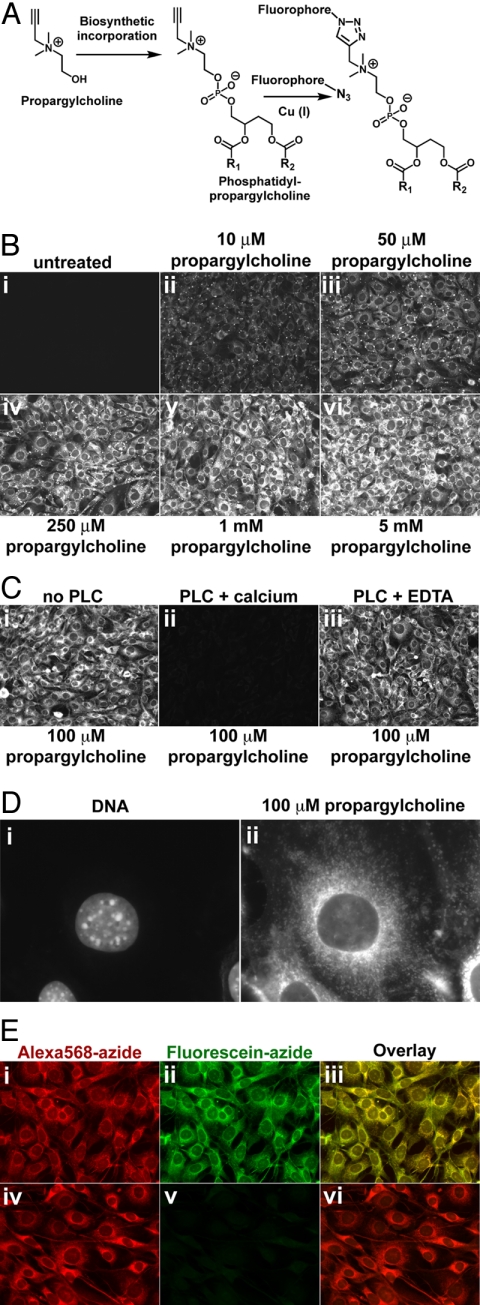
Fig. 1.
Imaging choline-containing phospholipids in cells with propargylcholine. (A) Propargyl-Cho, a biosynthetic label for Cho phospholipids. Phospholipid molecules bearing a terminal alkyne group can be detected by “click” chemistry using a fluorescent azide. (B) Propargyl-Cho incorporation into NIH 3T3 cells. Cells labeled overnight with varying concentrations of propargyl-Cho were fixed and stained with Alexa568-azide. Note the low background in the absence of propargyl-Cho (i) and the increase in staining intensity with increasing propargyl-Cho concentration (ii–vi). (C) Treatment of fixed cells with phospholipase C, which removes Cho head groups of phospholipids, strongly decreases propargyl-Cho staining by Alexa568-azide (compare ii to i). Phospholipase C requires calcium for activity; in the absence of calcium, propargyl-Cho staining is not affected by phospholipase C (iii). (D) Higher magnification fluorescence micrograph showing propargyl-Cho labeling of cellular organelles. (E) Reproducibility of propargyl-Cho staining. Cells labeled with 100 μM propargyl-Cho overnight were stained with 10 μM Alexa568-azide (i) and then with 20 μM fluorescein-azide (ii). Strong red and green signals indicate that the first staining reaction does not consume the incorporated propargyl-Cho. The red and green staining patterns are identical (iii), indicating that the two successive reactions detect the same phospholipid populations. The propargyl-Cho label can be consumed if, after Alexa568-azide (iv), cells are reacted with 5 mM non-fluorescent azide; this abolishes staining with fluorescein-azide (v and vi).