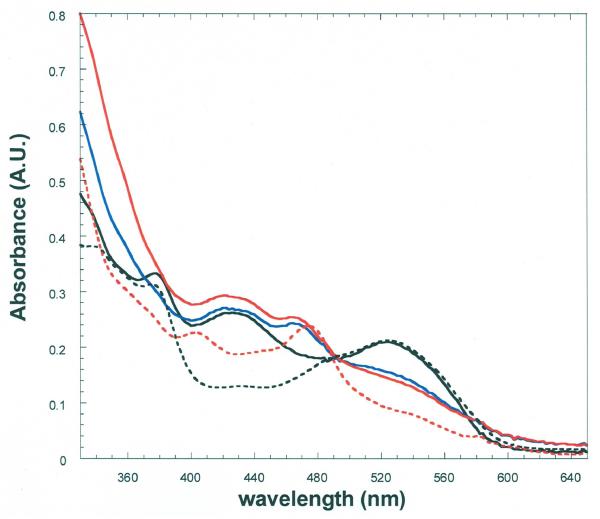
Figure 7.
Spectrophotometry in the reaction of 5,6-LAM with 4-thia-l-lysine.
The anaerobic solution contained 23 μM 5,6-LAM, 25 μM PLP, and 23 μM adenosylcobalamin in 100 mM K+EPPS buffer at pH 8.5, with 4-thia-l-lysine in a side arm. The solid-line spectra are: (—), 5,6-LAM-adenosylcobalamin-PLP before addition of 4-thia-l-lysine; (—), within 10 s of addition of 4-thia-l-lysine to 20 mM; (—), 30 min after addition of 4-thia-l-lysine. The dashed spectra are: (- - -), 5,6-LAM-adenosylcobalamin and (- - -), 5,6-LAM-cob(II)alamin after anaerobic photolysis of 5,6-LAM adenosylcobalamin. In the cleavage of adenosylcobalamin the 420 nm band is retained as that of the external PLP-4-thia-l-lysine aldimine.