Abstract
Identification of the active component and mechanisms of action of traditional medicines is highly desirable. We investigated whether zerumbone, a sesquiterpene from tropical ginger, can enhance the anticancer effects of TRAIL. We found that zerumbone potentiated TRAIL-induced apoptosis in human HCT116 colon cancer cells and that this was correlated with the up-regulation of TRAIL death receptor (DR)-4 and DR5. Induction of DRs occurred at the transcriptional level, and this induction was not cell type specific as its expression was also upregulated in prostate, kidney, breast, and pancreatic cancer cell lines. Deletion of DR5 or DR4 by siRNA significantly reduced the apoptosis induced by TRAIL and zerumbone. In addition to up-regulating DRs, zerumbone also significantly down-regulated the expression of cFLIP, but not that of other antiapoptotic proteins. The induction of both DRs by zerumbone was abolished by glutathione and N-acetylcysteine (NAC), and this correlated with decreased TRAIL-induced apoptosis, suggesting a critical role of reactive oxygen species (ROS). Inhibition of ERK1/2 and p38 MAPK but not of Jun N-terminal kinase abolished the effect of zerumbone on DR induction. Zerumbone also induced the p53 tumor suppressor gene but was found to be optional for DR induction or for enhancement of TRAIL-induced apoptosis. Both bax and p21, however, were required for zerumbone to stimulate TRAIL-induced apoptosis. Overall, our results demonstrate that zerumbone can potentiate TRAIL-induced apoptosis through the ROS-mediated activation of ERK1/2 and p38 MPAPK leading to DR4 and DR5 induction and resulting in enhancement of the anticancer effects of TRAIL.
Keywords: Zerumbone, TRAIL, death receptor, apoptosis, ROS
Introduction
Many traditional medicines have been used for thousands of years, and although they are safe and affordable, neither the active components nor the mechanisms of action are understood. Moreover, as many as 70% of all drugs approved by the U.S. Food and Drug Administration in the past 25 years have been based on natural products. Zerumbone, a sesquiterpene, was first isolated from the rhizome of a subtropical wild shampoo ginger (Zingiber zerumbet) and determined to be an anti-inflammatory agent (1). It was found to have anticancer activity against a wide variety of tumor cells, including colon cancer (1, 2), leukemia (3), myeloid cancer (4), and liver cancer (5), and to inhibit phorbol ester-induced Epstein-Barr virus activation (6). In vivo, this agent was found to suppress azoxymethane–induced colonic aberrant crypt foci in rats (2, 7), dextran sodium sulfate–induced colitis in mice (8), skin tumor initiation and promotion in mice (9), myeloid tumors in mice (4), cholecystokinin-induced acute pancreatitis in rats (10), CXCR4-mediated invasion of breast and pancreatic tumor cells (11), colon and lung carcinogenesis in mice (12), and human breast cancer-induced bone loss in nude mice (13). How zerumbone mediates all these effects is not understood, but suppression of constitutive and inducible nuclear factor (NF)-κB activation and NF-κB-regulated gene products has been demonstrated by different groups (12, 14). In contrast, zerumbone has been found to have very little or no cytotoxic effect on the normal human endothelial cells (3) and dermal fibroblasts (1). The cytotoxic effect of zerumbone on leukemia cells was found to be mediated through the induction of Fas receptors (3), leading to activation of caspase-8. Whether zerumbone has any effect on apoptosis mediated through other death receptor (DR) is not known.
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), a member of the TNF superfamily, is believed to regulate tumor cells killing through five different receptors, but only two of them, DR4 and DR5, are transmembrane receptors and mediate apoptosis. Engagement of DR4 and DR5 leads to the activation of caspase-8, which then leads to activation of caspase-9 and caspase-3 (15-17). The activation of caspase-8, also called FLICE, is inhibited by FLICE-interacting protein (cFLIP). The evidence that TRAIL may have an anticancer role also stems from the report that TRAIL deficiency accelerates the progression of hematological malignancies (18). Because of its selectivity toward tumor cells, both TRAIL and agonistic antibodies against its receptor are currently in clinical trials for cancer treatment in combination with various chemotherapeutic agents (17).
Some tumor cells have been shown to be resistant to TRAIL, and this resistance appears to be mediated through the loss of TRAIL receptors, enhanced expression of caspases inhibitors-such as cFLIP, X-linked inhibitor of apoptosis protein (XIAP), cIAP, and survivin-or alternations in expression of the Bcl-2 family proteins (19). Thus, agents that can up-regulate TRAIL receptors and down-regulate antiapoptotic proteins have the potential to enhance the apoptotic effects of TRAIL. In this study, we sought to determine the effect of zerumbone on TRAIL-induced apoptosis in colon cancer cells. Our results demonstrate that zerumbone can potentiate the apoptosis induced by TRAIL through the up-regulation of DR4 and DR5 expression and the down-regulation of cFLIP.
Materials and methods
Materials
Zerumbone, kindly supplied by Dr. Akira Murakami (Kyoto University, Kyoto, Japan). Soluble recombinant human TRAIL/Apo2L was purchased from PeproTech. Penicillin, streptomycin, Dulbecco's modified Eagle medium, RPMI 1640, and FBS were obtained from Invitrogen. All antibodies were obtained from Santa Cruz Biotechnology, BD Biosciences or Cell Signaling. siRNA for DR5, DR4 and ERK1 were synthesized or purchased from Qiagen.
Cell lines
Human cell lines HCT116 (colon adenocarcinoma), HT29 (colon adenocarcinoma), H1299 (lung adenocarcinoma), A293 (embryonic kidney carcinoma), PC3 (prostate adenocarcinoma) and DU145 (prostate adenocarcinoma), MDA-MB-231 (breast adenocarcinoma) and MCF-7 (breast adenocarcinoma), and AsPC1 (pancreatic adenocarcinoma) were obtained from American Type Culture Collection. HCT116 variants with deletions in p53, p21, and bax were kindly supplied by Dr. Bert Vogelstein (John Hopkins University). The colon cancer cells (HCT116 and HT29) were cultured in McCoy's 5A medium with 10% FBS and penicillin/streptomycin (Invitrogen). A293, MDA-MB-231, and MCF-7 were cultured in DMEM, and other cells lines were cultured in RPMI-1640 with 10% FBS and penicillin/streptomycin.
Live/Dead Assay
This assay was performed as described previously (14).
Cytotoxic assay
The effects of zerumbone on the cytotoxic effects of TRAIL agents were determined by the MTT uptake method as described (20).
Clonogenic Assay
Treated and untreated cells were seeded in 100-mm Petri dishes, allowed to form colonies for 14 days and then stained as described (21).
Analysis of cell surface expression of DR4 and DR5
Treated and untreated cells were stained with phycoerythrin -conjugated mouse monoclonal anti-human DR5 or DR4 (R&D Systems) for 45 min at 4°C according to manufacturer's instructions, resuspended and analyzed by flow cytometry with PE-conjugated mouse IgG2B as an isotype control.
Propidium iodide (PI) staining for DNA fragmentation
Cells were pretreated with zerumbone for 12 h and then exposed to TRAIL for 24 h. PI staining for DNA content analysis was performed as described elsewhere (22).
RNA analysis and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from the treated cells according to the manufacturer's instructions (Invitrogen) and performed RT-PCR as described (11).
Transfection with siRNA
HCT116 cells were plated in each well of 6-well plates and allowed to adhere for 24 h. On the day of transfection, 12 μL of Hiperfect transfection reagent (Qiagen) was added to 50 nmol/L siRNA in a final volume of 100 μL of culture medium. After 48 h of transfection, cells were treated with zerumbone for 12 h and then exposed TRAIL for 24 h.
Western blot analysis
To determine the levels of protein expression, we prepared whole-cell extracts and analyzed by Western blot as described previously (14). To measure activation of MAPK, whole-cell extracts from treated cells were subjected to Western blotting for phosphorylated ERK1/2, p38, and JNK. The same blots were stripped and reprobed with ERK1/2, p38, and JNK to ensure equal loading.
Results
The present studies were designed to investigate the effect of zerumbone on TRAIL-induced apoptosis. HCT116 colon cancer cells were used for most studies, but other cell types were also used to determine the specificity of this effect.
Zerumbone sensitizes colon cancer cells to TRAIL-mediated apoptosis
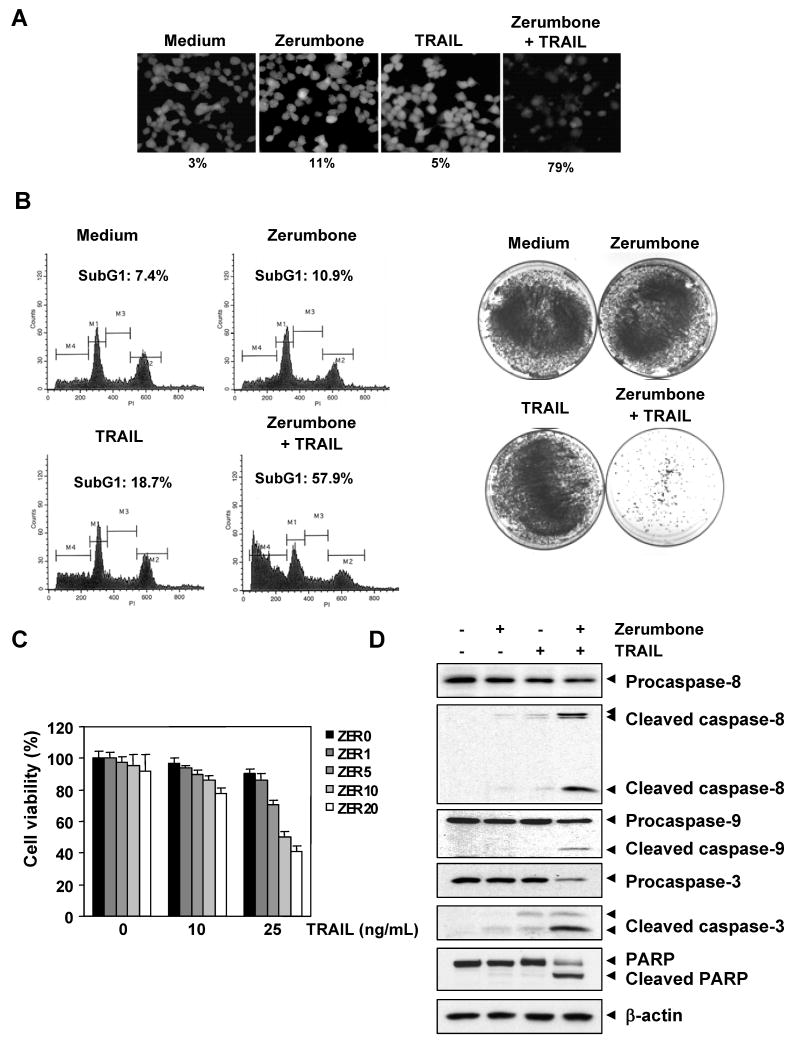
We determined whether zerumbone enhances TRAIL-induced apoptosis in HCT116 colon cancer cells. For this, cells were pretreated with zerumbone (20 μmol/L) for 12 h, washed with PBS to remove zerumbone, and then exposed to TRAIL (25 ng/mL) for 24 h. The results indicated that zerumbone and TRAIL treatment alone induced 11% and 5% apoptosis, respectively. Combination treatment with both zerumbone and TRAIL enhanced apoptosis to 79% (Fig. 1A). When apoptosis was examined using PI staining, we found that apoptosis was induced at 10.9% by zerumbone, 18.7% by TRAIL, and 57.9% by the combination of the two (Fig. 1B, left).
Figure 1.
Zerumbone enhances TRAIL-induced HCT116 cell death. (A) Cells were treated with 20 μmol/L zerumbone for 12 h and washed with PBS. Then cells were treated with 25 ng/mL TRAIL for 24 h. Cell death was determined using the Live/Dead Assay. (B) (Left) Cells were treated with zerumbone for 12 h and washed with PBS. Then cells were treated with 25 ng/mL TRAIL for 24 h. Cells were stained with PI, and the sub-G1 fraction was analyzed using flow cytometry. (Right) Cells were treated with zerumbone for 12 h, washed, and then treated with TRAIL (25 ng/mL) for 12 h. The cells were then reseeded in 100-mm dishes and incubated. After 14 days, cells were stained with crystal violet. (C) Cells were pretreated with various concentrations of zerumbone for 12 h, then medium was removed and cells exposed to TRAIL for 24 h. Cell viability was then analyzed by the MTT method. (D) Cells were pretreated with zerumbone for 12 h and washed out. Then cells were treated with TRAIL for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using antibodies against caspase-3, caspase-8, caspase-9, and PARP.
Whether zerumbone enhances the effect of TRAIL in long-term colony formation assay, was also examined. We found that under the conditions when zerumbone or TRAIL alone had minimal effect on colony formation of HCT116 cells, the combination treatment completely suppressed the colony-forming ability of these tumor cells (Fig. 1B, right).
We also examined the effect of zerumbone on TRAIL-induced cytotoxicity using the MTT method, which detects the mitochondrial activity. For this experiment, cells were pretreated with various concentrations of zerumbone for 12 h, and then exposed to TRAIL for 24 h. The HCT116 cells were moderately sensitive to either zerumbone or TRAIL alone. However, pretreatment with zerumbone enhanced TRAIL-induced cytotoxicity (Fig. 1C).
Next, we examined the effect of zerumbone, TRAIL, and the combination on the activation of caspase-8, caspase-9, caspase-3, and poly (ADP-ribose) polymerase (PARP) cleavage. We found that although zerumbone and TRAIL had little effect on the caspases and PARP, the two together were highly effective (Fig. 1D). Together our results indicate that zerumbone can enhance TRAIL-induced apoptosis.
Zerumbone induces the expression of TRAIL receptors DR4 and DR5 in cancer cell lines
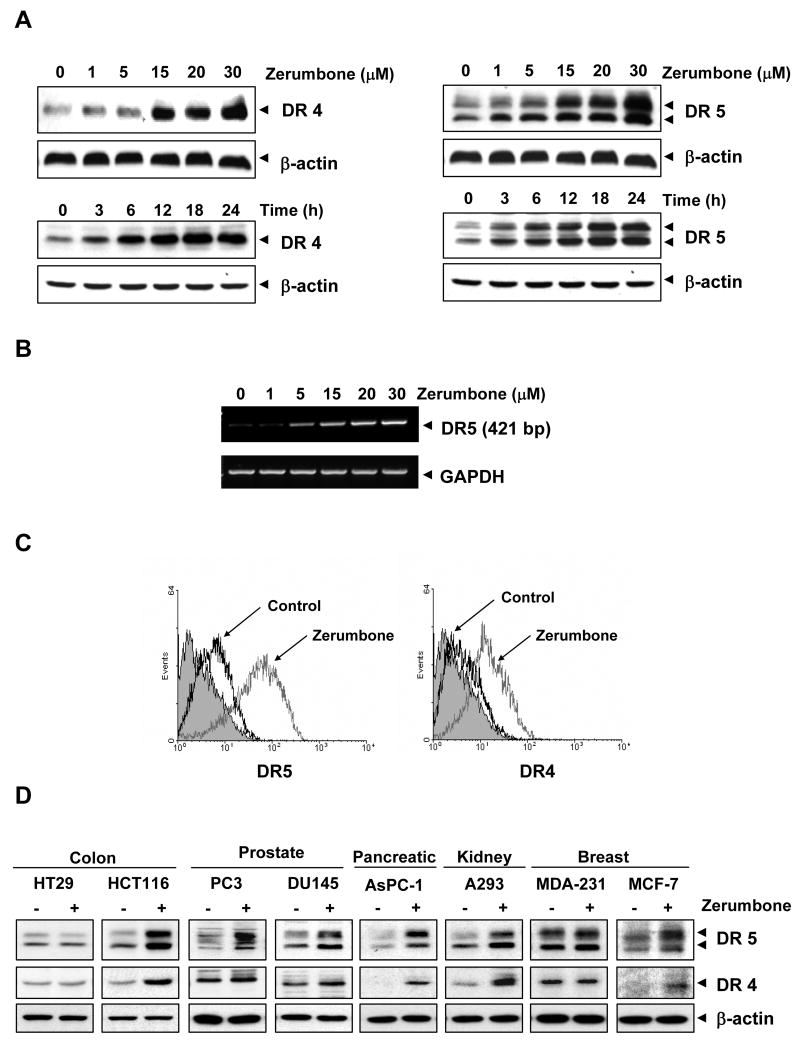
To determine how zerumbone potentiates TRAIL-induced apoptosis, we investigated its effect on TRAIL receptors (DR4 and DR5). For this, HCT116 cells were treated with different concentrations of zerumbone for 24 h, and whole-cell extracts were prepared and examined for expression of DR4 and DR5 proteins. Zerumbone induced both DR4 (Fig. 2A, left upper panel) and DR5 (Fig. 2A, right upper panel) in a dose-dependent manner, with optimum induction occurring at around 20-30 μmol/L. Whether this induction of the DRs was dependent on time was also examined. Zerumbone induced both DR4 (Fig. 2A, left lower panel) and DR5 (Fig. 2A, right lower panel) in a time-dependent manner.
Figure 2.
Zerumbone induced DR5 and DR4 expression. (A) HCT116 cells were treated with indicated doses of zerumbone at the indicated times. Whole-cell extracts were prepared and analyzed for DR4 and DR5 expression by Western blotting. (B) Zerumbone induced DR5 gene expression. HCT116 cells were treated with zerumbone for 24 h, and total RNA was extracted and examined for expression of DR5 by RT-PCR. GAPDH was used as an internal control to show equal RNA loading. (C) HCT116 cells were treated with 20 μmol/L zerumbone for 24 h and then harvested for analysis of cell surface DR4 and DR5 by immunofluorescent staining and subsequent flow cytometry. The filled gray peaks represented cells stained with a matched control PE-conjugated IgG isotype antibody. (D) Cells were treated with 20 μmol/L zerumbone for 24 h, whole-cell extracts were prepared, and analyzed by Western blotting using. Equal protein loading was evaluated by β-actin.
To determine whether induction of TRAIL receptors by zerumbone occurs at the transcriptional level, we examined mRNA for DR5 expression after cells had been treated with various concentrations of zerumbone. As shown in Fig. 2B, zerumbone induced the transcript for DR5 in a dose-dependent manner, thus suggesting that zerumbone acts at the transcriptional level.
Whether zerumbone enhances the expression of DRs on cell surface, was also examined. For this, we analyzed cell surface expression of DR5 and DR4 in cells exposed to zerumbone. We found that zerumbone increased cell surface levels of DR5 and DR4 (Fig. 2C). The level of DR4 cell surface expression induced by zerumbone, was lower than that of DR5. Collectively, these results indicate that zerumbone up-regulated the expression of both DRs on the cell surface.
We also investigated whether up-regulation of DR5 and DR4 by zerumbone was specific to HCT116 or also occurs in other cell types. For this, colon cancer cells (HT29 and HCT116), prostate cancer cells (PC3 and DU145), pancreatic cancer cells (AsPC-1), embryonic kidney cells (A293), and breast cancer cells (MDA-MB-231 and MCF-7) were exposed to zerumbone (20 μmol/L) for 24 h and then examined for DR5 and DR4 protein expression. Zerumbone induced the expression of DR5 (Fig. 2D, top panel) in HCT116, PC3, DU145, AsPC-1, A293, and MCF-7 cells. The sesquiterpene induced the expression of DR4 (Fig. 2D, middle panel) in HCT116, AsPC-1, A293, and MCF-7 cells. No significant induction of DR4 was noted in prostate cancer cell lines PC3 and DU145 (Fig. 2D). Induction of either DR4 or DR5 was minimal in breast cancer MDA-MB-231 and colon cancer HT29 cells after exposure to zerumbone. Together, these findings suggest that the up-regulation of DR5 and DR4 by zerumbone is not cell type specific.
DR induction by zerumbone is needed for TRAIL-induced apoptosis
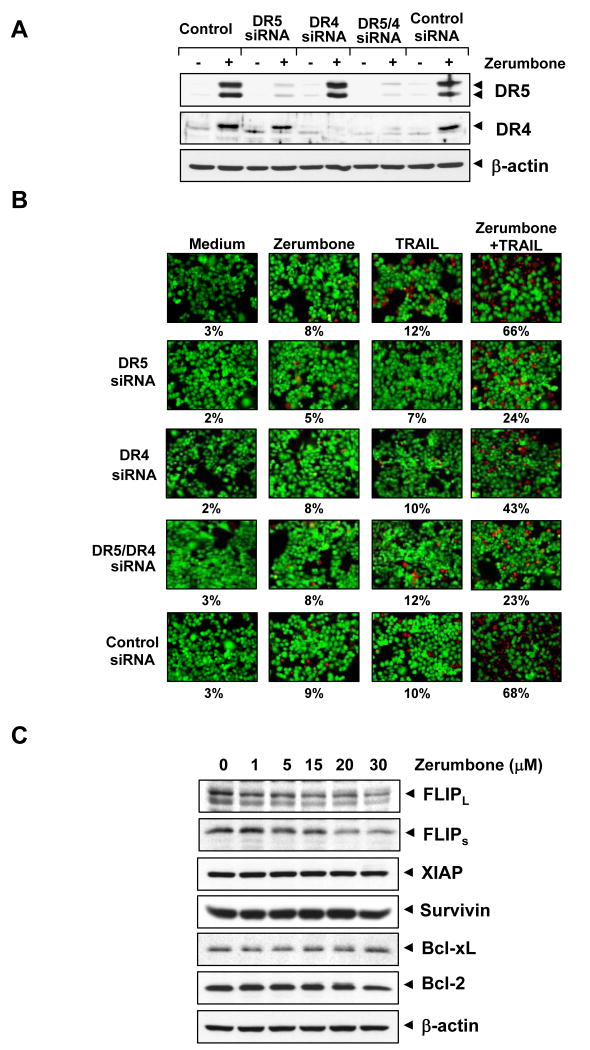
To determine the role of DR5 and DR4 in TRAIL-induced apoptosis, we used siRNA specific to DR5 and DR4 to down-regulate the expression of these receptors. Transfection of cells with siRNA for DR5 but not with the control siRNA reduced zerumbone-induced DR5 expression (Fig. 3A). Similarly, transfection of cells with siRNA for DR4 reduced the zerumbone-induced DR4 expression (Fig. 3A). However, DR4 siRNA had minimal effect on the zerumbone-induced up-regulation of DR5.
Figure 3.
Effects of knockdown of DRs on zerumbone-induced sensitization of TRAIL. (A) HCT116 cells were transfected with DR5 siRNA, DR4 siRNA, and control siRNA alone, or combined. After 48 h, cells were treated with 20 μmol/L zerumbone for 24 h, and whole-cell extracts were subjected to Western blotting for DR5 and DR4. (B) Cells were seeded in a chamber slide and transfected with siRNAs. After 48 h, cells were pretreated with 20 μmol/L zerumbone for 12 h and then incubated with 25 ng/mL TRAIL for 24 h. Cell death was determined by the Live/Dead Assay. (C) Effects of zerumbone on antiapoptotic protein expression. HCT116 cells were pretreated with the indicated dose of zerumbone for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using relevant antibodies.
We next examined whether the suppression of DR5 or DR4 by siRNA could abrogate the sensitizing effects of zerumbone on TRAIL-induced apoptosis using an esterase staining assay (the Live/Dead Assay). The results reveal that the effect of zerumbone on TRAIL-induced apoptosis was effectively abolished in cells transfected with either DR5 or DR4 siRNA (Fig. 3B), whereas treatment with control siRNA had no effect (Fig. 3B). Silencing of DR5 had more dramatic effect on TRAIL-induced apoptosis than that of DR4. The silencing of both the receptors abolished the apoptosis as much as silencing of DR5 alone, thus suggesting that DR5 is a major player in TRAIL-induced apoptosis.
Zerumbone down-regulates the expression of cFLIP but has no effect on XIAP, survivin, and Bcl-2 family proteins
We examined whether zerumbone has any effect on the expression of any of the anti-apoptotic proteins. Cells were exposed to different concentrations of zerumbone for 24 h and then examined for expression of cFLIP, XIAP, survivin, Bcl-xL, and Bcl-2 expression. Zerumbone down-regulated the expression of both forms of cFLIP but had little effect on the other anti-apoptotic proteins (Fig. 3C). Thus, it is possible that zerumbone affects TRAIL-induced apoptosis not only through induction of DR4 and DR5 but also through the down-regulation of cFLIP, an inhibitor of caspase-8.
Zerumbone-induced up-regulation of TRAIL receptors is dependent on reactive oxygen species (ROS)
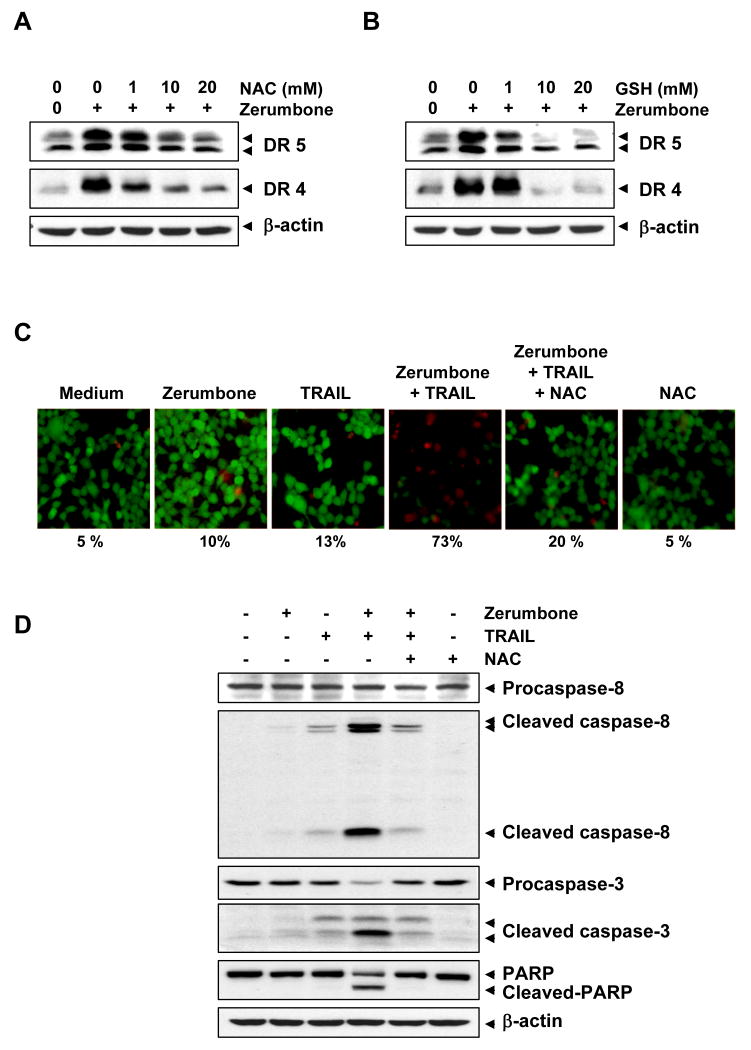
There has been a report that TRAIL-induced apoptosis is regulated by ROS (23). Another study suggested that induction of DR5 by agents such as curcumin requires ROS and exposure of renal cancer cells to peroxide induces DR5 (24). We sought to determine whether zerumbone-induced TRAIL receptors are also regulated by ROS. As shown in Fig. 4A, pre-treating HCT116 cells with the ROS scavenger NAC reduced the zerumbone-induced up-regulation of both DR5 and DR4 expression in a dose-dependent manner. We also examined the effect of glutathione (GSH) on the zerumbone-induced expression of TRAIL receptors and found that GSH pretreatment abolished the induction of DR4 and DR5 by zerumbone in a dose-dependent manner (Fig. 4B). This suggests the critical role of ROS in the induction of TRAIL receptors by zerumbone.
Figure 4.
Up-regulation of DR4 and DR5 by zerumbone was mediated by ROS. HCT116 cells were pretreated with various concentrations of NAC (A) or GSH (B) for 1 h, then the cells were treated with 20 μmol/L zerumbone for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using DR5 and DR4 antibodies. (C) NAC reversed cell death induced by the combination of zerumbone and TRAIL. HCT116 cells were pretreated with NAC for 1 h and then treated with zerumbone for 12 h. Next, cells were washed with PBS and treated with TRAIL for 24 h. Cell death was determined by the Live/Dead Assay. (D) NAC suppressed caspase activation and PARP cleavage induced by combination of zerumbone and TRAIL. HCT116 cells were pretreated with NAC for 1 h and then treated with zerumbone for 12 h. After then, cells were washed with PBS and treated with TRAIL for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using relevant antibodies. β-actin was used as a loading control.
We next examined whether scavenging of ROS could attenuate the TRAIL-induced cell death enhanced by zerumbone. As shown in Fig. 4C, zerumbone significantly enhanced TRAIL-induced apoptosis in colon cancer cells, and pretreatment of cells with NAC markedly reduced this zerumbone-induced enhancement, from 73% to 20%.
Then we determined whether NAC pretreatment also attenuates the zerumbone-induced increase in activation of caspase-8, caspase-3, and PARP cleavage (Fig. 4D). We found that zerumbone enhanced TRAIL-induced cleavage of procaspase-8, procaspase-3, and PARP and that NAC abolished this increase, again suggesting the critical role of ROS.
Up-regulation of TRAIL receptors by zerumbone is reversed by inhibitors of mitogen-activated protein kinases (MAPKs)
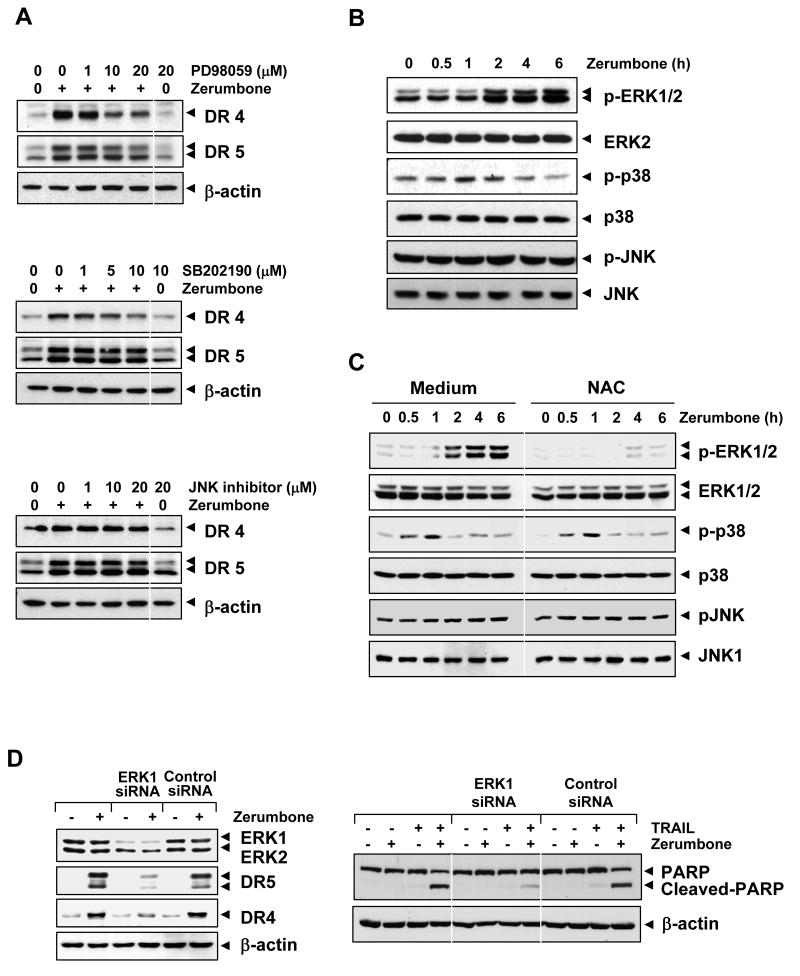
MAPKs, including extracellular signal-regulated kinases (ERK)1/2, p38 MAPK, and Jun N-terminal kinase (JNK), have been shown to mediate the up-regulation of DR5 by bisindolylmaleimide and LY303511 (25, 26). We investigated whether these MAPKs have any role in zerumbone-induced TRAIL receptors. As shown in Fig. 5A, pretreatment of cells with an ERK1/2 inhibitor (PD98059) suppressed the zerumbone-induced up-regulation of DR4 and DR5 in a dose-dependent manner.
Figure 5.
(A) Up-regulation of DR4 and DR5 by zerumbone was mediated through the activation of MAPK pathway. Cells were pretreated with various concentrations of PD98059, SB202190, and JNK inhibitor for 1 h and then treated with 20 μmol/L zerumbone for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using DR4 and DR5 antibodies. (B) Zerumbone activated ERK. Cells were treated with 20 μmol/L zerumbone and whole-cell extracts were subjected to Western blotting for phosphor-ERK1/2, -p38, and -JNK. The same blots were stripped and reprobed with ERK1/2, p38, and JNK to ensure equal loading. (C) Zerumbone-induced ERK activation is dependent on ROS. Cell were pretreated with NAC (20 mmol/L) for 1 h and then exposed to 20 μmol/L zerumbone for the indicated times. Whole-cell extracts were prepared and subjected to Western blotting using relevant antibodies. (D) Blockade of ERK reversed effect of zerumbone on TRAIL-mediated cell death. Cells were transfected with either ERK1 siRNA, or control siRNA. After 48 h, cells were treated with 20 μmol/L zerumbone for 24 h, and whole-cell extracts were subjected to Western blotting (left). (Right) Cells and siRNA transfected cells were pretreated with 20 μmol/L zerumbone for 12 h and then incubated with 25 ng/mL TRAIL for further 24 h. Cell death was determined by the PAPR cleavage. Equal protein loading was evaluated by β-actin.
Pretreatment of cells with a p38 inhibitor (SB202190) also reduced zerumbone-induced DR4 up-regulation but to a lesser extent (Fig. 5A; middle panel). No effect of the p38 inhibitor was observed on zerumbone-induced DR4 and DR5 expression. Compared with inhibitors of ERK and p38 MAPK, JNK inhibitor had no effect on zerumbone-induced DR4 or DR5 expression (Fig. 5A, bottom panel).
We also examined whether zerumbone can activate ERK, p38 MAPK, and JNK. We found that zerumbone activated ERK in a time-dependent manner (Fig. 5B, upper panel). Zerumbone activated p38 MAPK transitorily between 1 and 2 h and declined thereafter (Fig. 5B, middle panel). No effect of zerumbone was observed on the activation of JNK. Thus, the activation of these various MAPKs is consistent with the results obtained with the effect of their inhibitors on the zerumbone-induced expression of TRAIL receptors.
Zerumbone-induced activation of MAPK is dependent on ROS
So far, our data indicated that ERK and ROS are involved in expression of DR by zerumbone. Whether zerumbone-induced activation of MPAK, is also dependent on ROS production was examined. To do this, cells were exposed to antioxidant NAC (20 mmol/L) for 1 h, then treated with zerumbone (30 μmol/L) for indicated times and examined for MAPK activation. We found that zerumbone activated ERK and the presence of NAC inhibited the zerumbone-induced phosphorylation of ERK but had no effect on activation of p38 MAPK and JNK (Fig. 5C). These results indicate that zerumbone-induced activation of MAPK is dependent on ROS.
Silencing of ERK blocks death receptor upregulation and TRAIL sensitization
We next determined whether silencing of ERK blocks death receptor upregulation and inhibits TRAIL sensitization. To examine this, HCT116 cells were transfected with control and specific siRNA against ERK1. After 48 h, cells were then exposed to zerumbone for 24h, harvested, and lysates were subjected to Western blotting. The result indicate that the expression of ERK1 was significantly reduced in cells treated specific siRNA when compared with cells left untreated or treated with control siRNA (Fig. 5A, left panel). The reduction in expression of ERK1 by the siRNA, correlated with suppression of zerumbone-induced up-regulation of DR5 and DR4 (Fig. 5A, left panel).
Furthermore, siERK1 significantly inhibited the enhancement of TRAIL-induced PARP cleavage by zerumbone. However, control siRNA had no effect (Fig. 5D, right panel).
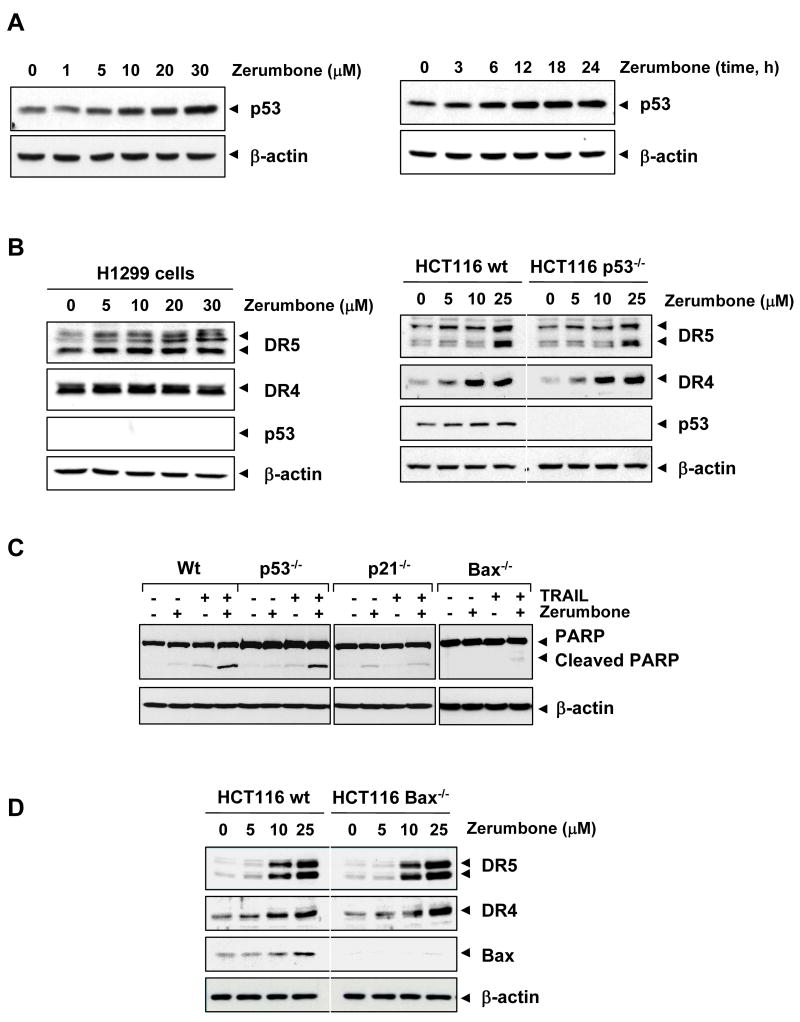
Zerumbone can induce p53 but is not required for expression of TRAIL receptors
Numerous reports have suggested that p53 can induce death receptors (27, 28). We examined whether zerumbone can induce p53 and, if so, whether this action mediates the induction of TRAIL receptors. As shown in Fig. 6A, zerumbone induced p53 expression in a dose- and time-dependent manner.
Figure 6.
(A) Zerumbone increased p53 expression. HCT116 cells were treated with various concentration and times as indicated. Whole-cell extracts were prepared and analyzed by Western blotting using p53 antibody. (B) Effect of p53 on zerumbone-induced DR expression. H1299 cells (p53 null cells; left) and HCT116 and p53 variant cells (right) were treated with zerumbone for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using relevant antibodies. (C) Cells were pretreated with zerumbone for 12 h and exposed to TRAIL for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using indicated antibodies. (D) HCT116 and bax variant cells were treated with zerumbone for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using relevant antibodies. Equal protein loading was evaluated by β-actin.
To determine whether p53 is needed for zerumbone-induced DRs induction, we used H1299 lung cancer cells that are deficient in p53 protein expression. As shown in Fig. 6B (left), zerumbone induced DR5 but not DR4 in p53-deficient H1299 cells, even though these cells do not express p53 protein. To clarify the difference between results using colon and lung cancer cell lines, we also used an HCT116 variant in which p53 is deleted. Again, we found that zerumbone induced DR5 as well as DR4 in both wild-type and p53-deleted cells, thus indicating that p53 has no role in the induction of either DR by zerumbone (Fig. 6B, right).
To determine whether deletion of p53 modulates the apoptotic effect of zerumbone on TRAIL-induced apoptosis, we examined PARP cleavage. Enhanced apoptosis was observed when TRAIL was combined with zerumbone in wild-type cells, but this was unaffected in p53-deleted cells, suggesting that p53 induction plays little role in TRAIL-induced apoptosis (Fig. 6C; left panel). The enhancement of apoptosis by zerumbone, however, was abolished in cells in which either p21 (Fig. 6C, middle panel) or bax was deleted (Fig. 6C, right panel), suggesting the critical role of these two proteins.
Deletion of Bax has no effect on upregulation of DR by zerumbone
Whether the deletion of bax modulates the expression of DR by zerumbone in HCT116 cells, was examined. The effect of bax on the expression of DR5 and DR4 induced by zerumbone, was investigated using bax-deleted cells. Results show that zerumbone increased the expression of bax, DR5 and DR4 in wild-type cells but deletion of bax had no effect on the induction of these receptors by zerumbone (Fig. 6D). These results suggest that bax is not linked to the upregulation of DR expression by zerumbone.
Discussion
In the present report we demonstrate that zerumbone, a component of wild ginger, can enhance the apoptotic effects of TRAIL against colon cancer cells. The mechanism by which zerumbone mediates its effects on TRAIL-induced apoptosis appears to involve the induction of TRAIL receptors and down-regulation of cFLIP, an inhibitor of caspase-8. We found that the induction of DRs by zerumbone was not cell type specific but was observed in a wide variety of cell types, including colon, breast, prostate, kidney, and pancreatic cancer cells. Induction of TRAIL receptors in some cells, however, was much more pronounced than that in other cell types. We found that zerumbone had no effect on human breast cancer MDA-MB-231 and colon cancer HT29 cells. Both of these cell lines are known to express mutant p53 (29, 30). Our results, however, show that the lack of induction of receptors by zerumbone is not due to mutation in p53. The mechanism by which this sesquiterpene induces DR-5 appears to occur at the transcriptional level. We also found that zerumbone induces TRAIL receptors through the generation of ROS, as shown by our results that NAC and GSH abolished the induction of DRs. The results regarding the induction of DRs by zerumbone through ROS are consistent with those in previous reports regarding curcumin (24), inactive PI3K inhibitor LY303511 (26), proteasome inhibitors (28), H2O2 (31), baicalein (32), and 15-deoxy-delta(12,14)-prostaglandin J2 (33). We found that an increase in TRAIL-induced apoptosis by zerumbone was also reversed by NAC and GSH. Apoptosis induced by TRAIL alone is also known to be regulated in colon cancer cells through the generation of ROS (23).
Besides the induction of DRs, the down-regulation of cFLIP by zerumbone may also lead to the enhancement of TRAIL-induced apoptosis. Zerumbone was found to have little effect on other antiapoptotic proteins, including XIAP, survivin, Bcl-2, and Bcl-xL. Interestingly, like zerumbone, a sesquiterpene, the synthetic triterpenoids 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO) and its methyl ester analogue (CDDO-m) have also been shown to down-regulate the expression of cFLIP and to enhance TRAIL-induced apoptosis (34, 35). In addition to terpenoids, celecoxib and honokiol enhance TRAIL-induced apoptosis through the down-regulation of cFLIP (36, 37).
We found that the induction of DR4 and DR5 by zerumbone required the activation of ERK1/2 and MAPK, as demonstrated by our results that zerumbone activated these kinases and that their inhibitors abolished zerumbone-induced expression of TRAIL receptors. Given that ROS induces MAPK (26, 38, 39), it is possible that zerumbone induces TRAIL receptors through the sequential induction of ROS and MAPK. In contrast to ERK, p38 MAPK was found to be involved in induction of DR4 but not that of DR5. JNK was found to have no role in the induction of TRAIL receptors by zerumbone. That activation of ERK1/2 by Ras can lead to the up-regulation of DR4 and DR5 has been previously demonstrated (40). In addition, quercetin has been shown to augment TRAIL-induced apoptosis through the ERK-mediated down-regulation of the survivin signal transduction pathway (41). In our studies, however, we found no effect of zerumbone on the expression of survivin.
The tumor suppressor gene p53 is a key regulator of apoptosis in cancer cells. We found that zerumbone induced p53 protein expression. Although p53 expression has been implicated in the expression of DR5 (28, 42), we found that zerumbone-induced DR5 expression was mediated through a p53-independent mechanism. No induction of DR4, however, was observed in p53-null cells. That p53 plays a role in the up-regulation of DR4 has already been shown (43). Thus it is possible that the role of p53 in the induction of DR4 and DR5 depends on the nature of the stimulus and the cell type. We found that zerumbone's ability to enhance TRAIL-induced apoptosis was unaffected by a lack of p53 expression in cells.
Overall, we conclude that zerumbone can enhance TRAIL-induced apoptosis through the up-regulation of DRs and the down-regulation of cFLIP. Considering that zerumbone when used alone is highly safe and exhibits anticancer activities in vitro (1-5) and in vivo (1, 2, 7, 9, 12) against a wide variety of tumors, we suggest that it can be also used in combination with TRAIL that is in clinical trials currently. Thus these studies have an implication in treatment of cancer by TRAIL, especially tumors that develop resistance to TRAIL.
Acknowledgments
We thank Ms. Virginia M. Mohlere for carefully proof-reading the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from the Clayton Foundation for Research (B.B.A.), a core grant from the National Institutes of Health (CA-16 672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), and a grant from Center for Targeted Therapy of The University of Texas M. D. Anderson Cancer Center.
References
- 1.Murakami A, Takahashi D, Kinoshita T, et al. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: the alpha,beta-unsaturated carbonyl group is a prerequisite. Carcinogenesis. 2002;23:795–802. doi: 10.1093/carcin/23.5.795. [DOI] [PubMed] [Google Scholar]
- 2.Kirana C, McIntosh GH, Record IR, Jones GP. Antitumor activity of extract of Zingiber aromaticum and its bioactive sesquiterpenoid zerumbone. Nutr Cancer. 2003;45:218–25. doi: 10.1207/S15327914NC4502_12. [DOI] [PubMed] [Google Scholar]
- 3.Xian M, Ito K, Nakazato T, et al. Zerumbone, a bioactive sesquiterpene, induces G2/M cell cycle arrest and apoptosis in leukemia cells via a Fas- and mitochondria-mediated pathway. Cancer Sci. 2007;98:118–26. doi: 10.1111/j.1349-7006.2006.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang GC, Chien TY, Chen LG, Wang CC. Antitumor effects of zerumbone from Zingiber zerumbet in P-388D1 cells in vitro and in vivo. Planta Med. 2005;71:219–24. doi: 10.1055/s-2005-837820. [DOI] [PubMed] [Google Scholar]
- 5.Sakinah SA, Handayani ST, Hawariah LP. Zerumbone induced apoptosis in liver cancer cells via modulation of Bax/Bcl-2 ratio. Cancer Cell Int. 2007;7:4. doi: 10.1186/1475-2867-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami A, Takahashi M, Jiwajinda S, Koshimizu K, Ohigashi H. Identification of zerumbone in Zingiber zerumbet Smith as a potent inhibitor of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation. Biosci Biotechnol Biochem. 1999;63:1811–2. doi: 10.1271/bbb.63.1811. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka T, Shimizu M, Kohno H, et al. Chemoprevention of azoxymethane-induced rat aberrant crypt foci by dietary zerumbone isolated from Zingiber zerumbet. Life Sci. 2001;69:1935–45. doi: 10.1016/s0024-3205(01)01277-2. [DOI] [PubMed] [Google Scholar]
- 8.Murakami A, Hayashi R, Tanaka T, Kwon KH, Ohigashi H, Safitri R. Suppression of dextran sodium sulfate-induced colitis in mice by zerumbone, a subtropical ginger sesquiterpene, and nimesulide: separately and in combination. Biochem Pharmacol. 2003;66:1253–61. doi: 10.1016/s0006-2952(03)00446-5. [DOI] [PubMed] [Google Scholar]
- 9.Murakami A, Tanaka T, Lee JY, et al. Zerumbone, a sesquiterpene in subtropical ginger, suppresses skin tumor initiation and promotion stages in ICR mice. Int J Cancer. 2004;110:481–90. doi: 10.1002/ijc.20175. [DOI] [PubMed] [Google Scholar]
- 10.Szabolcs A, Tiszlavicz L, Kaszaki J, et al. Zerumbone exerts a beneficial effect on inflammatory parameters of cholecystokinin octapeptide-induced experimental pancreatitis but fails to improve histology. Pancreas. 2007;35:249–55. doi: 10.1097/mpa.0b013e318070d791. [DOI] [PubMed] [Google Scholar]
- 11.Sung B, Jhurani S, Ahn KS, et al. Zerumbone down-regulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res. 2008;68:8938–44. doi: 10.1158/0008-5472.CAN-08-2155. [DOI] [PubMed] [Google Scholar]
- 12.Kim M, Miyamoto S, Yasui Y, Oyama T, Murakami A, Tanaka T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int J Cancer. 2009;124:264–71. doi: 10.1002/ijc.23923. [DOI] [PubMed] [Google Scholar]
- 13.Sung B, Murakami A, Oyajobi BO, Aggarwal BB. Zerumbone abolishes RANKL-induced NF-kappaB activation, inhibits osteoclastogenesis, and suppresses human breast cancer-induced bone loss in athymic nude mice. Cancer Res. 2009;69:1477–84. doi: 10.1158/0008-5472.CAN-08-3249. [DOI] [PubMed] [Google Scholar]
- 14.Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-kappaB and IkappaBalpha kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24:6957–69. doi: 10.1038/sj.onc.1208845. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity. 1997;7:821–30. doi: 10.1016/s1074-7613(00)80400-8. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–56. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 17.Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26:3621–30. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 18.Zerafa N, Westwood JA, Cretney E, et al. Cutting edge: TRAIL deficiency accelerates hematological malignancies. J Immunol. 2005;175:5586–90. doi: 10.4049/jimmunol.175.9.5586. [DOI] [PubMed] [Google Scholar]
- 19.Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001;21:8247–54. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Takada Y, Sethi G, Sung B, Aggarwal BB. Flavopiridol suppresses tumor necrosis factor-induced activation of activator protein-1, c-Jun N-terminal kinase, p38 mitogen-activated protein kinase (MAPK), p44/p42 MAPK, and Akt, inhibits expression of antiapoptotic gene products, and enhances apoptosis through cytochrome c release and caspase activation in human myeloid cells. Mol Pharmacol. 2008;73:1549–57. doi: 10.1124/mol.107.041350. [DOI] [PubMed] [Google Scholar]
- 22.Pathak AK, Bhutani M, Nair AS, et al. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res. 2007;5:943–55. doi: 10.1158/1541-7786.MCR-06-0348. [DOI] [PubMed] [Google Scholar]
- 23.Izeradjene K, Douglas L, Tillman DM, Delaney AB, Houghton JA. Reactive oxygen species regulate caspase activation in tumor necrosis factor-related apoptosis-inducing ligand-resistant human colon carcinoma cell lines. Cancer Res. 2005;65:7436–45. doi: 10.1158/0008-5472.CAN-04-2628. [DOI] [PubMed] [Google Scholar]
- 24.Jung EM, Lim JH, Lee TJ, Park JW, Choi KS, Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5) Carcinogenesis. 2005;26:1905–13. doi: 10.1093/carcin/bgi167. [DOI] [PubMed] [Google Scholar]
- 25.Ohtsuka T, Zhou T. Bisindolylmaleimide VIII enhances DR5-mediated apoptosis through the MKK4/JNK/p38 kinase and the mitochondrial pathways. J Biol Chem. 2002;277:29294–303. doi: 10.1074/jbc.M203342200. [DOI] [PubMed] [Google Scholar]
- 26.Shenoy K, Wu Y, Pervaiz S. LY303511 enhances TRAIL sensitivity of SHEP-1 neuroblastoma cells via hydrogen peroxide-mediated mitogen-activated protein kinase activation and up-regulation of death receptors. Cancer Res. 2009;69:1941–50. doi: 10.1158/0008-5472.CAN-08-1996. [DOI] [PubMed] [Google Scholar]
- 27.Tomasetti M, Andera L, Alleva R, Borghi B, Neuzil J, Procopio A. Alpha-tocopheryl succinate induces DR4 and DR5 expression by a p53-dependent route: implication for sensitisation of resistant cancer cells to TRAIL apoptosis. FEBS Lett. 2006;580:1925–31. doi: 10.1016/j.febslet.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 28.Chen JJ, Chou CW, Chang YF, Chen CC. Proteasome inhibitors enhance TRAIL-induced apoptosis through the intronic regulation of DR5: involvement of NF-kappa B and reactive oxygen species-mediated p53 activation. J Immunol. 2008;180:8030–9. [Google Scholar]
- 29.Watson NC, Di YM, Orr MS, et al. Influence of ionizing radiation on proliferation, c-myc expression and the induction of apoptotic cell death in two breast tumour cell lines differing in p53 status. Int J Radiat Biol. 1997;72:547–59. doi: 10.1080/095530097143059. [DOI] [PubMed] [Google Scholar]
- 30.Hiro J, Inoue Y, Toiyama Y, Miki C, Kusunoki M. Mechanism of resistance to chemoradiation in p53 mutant human colon cancer. Int J Oncol. 2008;32:1305–10. doi: 10.3892/ijo_32_6_1305. [DOI] [PubMed] [Google Scholar]
- 31.Kwon D, Choi K, Choi C, Benveniste EN. Hydrogen peroxide enhances TRAIL-induced cell death through up-regulation of DR5 in human astrocytic cells. Biochem Biophys Res Commun. 2008;372:870–4. doi: 10.1016/j.bbrc.2008.05.148. [DOI] [PubMed] [Google Scholar]
- 32.Taniguchi H, Yoshida T, Horinaka M, et al. Baicalein overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance via two different cell-specific pathways in cancer cells but not in normal cells. Cancer Res. 2008;68:8918–27. doi: 10.1158/0008-5472.CAN-08-1120. [DOI] [PubMed] [Google Scholar]
- 33.Su RY, Chi KH, Huang DY, Tai MH, Lin WW. 15-deoxy-Delta12,14-prostaglandin J2 up-regulates death receptor 5 gene expression in HCT116 cells: involvement of reactive oxygen species and C/EBP homologous transcription factor gene transcription. Mol Cancer Ther. 2008;7:3429–40. doi: 10.1158/1535-7163.MCT-08-0498. [DOI] [PubMed] [Google Scholar]
- 34.Hyer ML, Croxton R, Krajewska M, et al. Synthetic triterpenoids cooperate with tumor necrosis factor-related apoptosis-inducing ligand to induce apoptosis of breast cancer cells. Cancer Res. 2005;65:4799–808. doi: 10.1158/0008-5472.CAN-04-3319. [DOI] [PubMed] [Google Scholar]
- 35.Suh WS, Kim YS, Schimmer AD, et al. Synthetic triterpenoids activate a pathway for apoptosis in AML cells involving downregulation of FLIP and sensitization to TRAIL. Leukemia. 2003;17:2122–9. doi: 10.1038/sj.leu.2403112. [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Liu X, Yue P, Schonthal AH, Khuri FR, Sun SY. CCAAT/enhancer binding protein homologous protein-dependent death receptor 5 induction and ubiquitin/proteasome-mediated cellular FLICE-inhibitory protein down-regulation contribute to enhancement of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by dimethyl-celecoxib in human non small-cell lung cancer cells. Mol Pharmacol. 2007;72:1269–79. doi: 10.1124/mol.107.037465. [DOI] [PubMed] [Google Scholar]
- 37.Raja SM, Chen S, Yue P, et al. The natural product honokiol preferentially inhibits cellular FLICE-inhibitory protein and augments death receptor-induced apoptosis. Mol Cancer Ther. 2008;7:2212–23. doi: 10.1158/1535-7163.MCT-07-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MW, Park SC, Yang YG, et al. The involvement of reactive oxygen species (ROS) and p38 mitogen-activated protein (MAP) kinase in TRAIL/Apo2L-induced apoptosis. FEBS Lett. 2002;512:313–8. doi: 10.1016/s0014-5793(02)02225-1. [DOI] [PubMed] [Google Scholar]
- 39.Huang J, Wu L, Tashiro S, Onodera S, Ikejima T. Reactive oxygen species mediate oridonin-induced HepG2 apoptosis through p53, MAPK, and mitochondrial signaling pathways. J Pharmacol Sci. 2008;107:370–9. doi: 10.1254/jphs.08044fp. [DOI] [PubMed] [Google Scholar]
- 40.Drosopoulos KG, Roberts ML, Cermak L, et al. Transformation by oncogenic RAS sensitizes human colon cells to TRAIL-induced apoptosis by up-regulating death receptor 4 and death receptor 5 through a MEK-dependent pathway. J Biol Chem. 2005;280:22856–67. doi: 10.1074/jbc.M412483200. [DOI] [PubMed] [Google Scholar]
- 41.Kim JY, Kim EH, Park SS, Lim JH, Kwon TK, Choi KS. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. J Cell Biochem. 2008;105:1386–98. doi: 10.1002/jcb.21958. [DOI] [PubMed] [Google Scholar]
- 42.Wu GS, Burns TF, McDonald ER, 3rd, et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet. 1997;17:141–3. doi: 10.1038/ng1097-141. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Yue P, Khuri FR, Sun SY. p53 upregulates death receptor 4 expression through an intronic p53 binding site. Cancer Res. 2004;64:5078–83. doi: 10.1158/0008-5472.CAN-04-1195. [DOI] [PubMed] [Google Scholar]