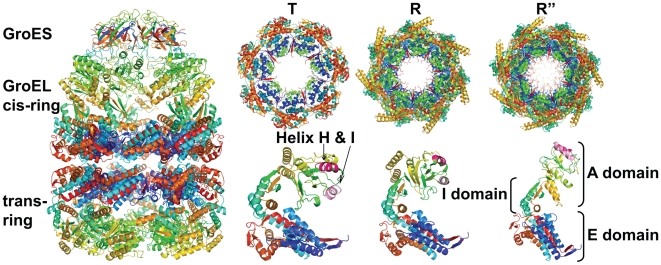
Figure 1. The structure of GroEL-GroES chaperone complex.
Side view for the R″ state (left), top view (upper right) and side view (lower right) for the T, R and R″ state (pdb ids  ,
,  and
and  , respectively). The GroEL-GroES chaperone complex is large, consisting of 8,015 residues in the resolved structure, with 14 and 7 chains in the homo-oligemeric GroEL and GroES subunits, respectively. Each chain in the GroEL structure contains three domains: the E (equatorial) domain, the I (intermediate) domain, and the A (apical) domain, reflecting their respective spatial positions in the GroEL-GroES chaperone complex.
, respectively). The GroEL-GroES chaperone complex is large, consisting of 8,015 residues in the resolved structure, with 14 and 7 chains in the homo-oligemeric GroEL and GroES subunits, respectively. Each chain in the GroEL structure contains three domains: the E (equatorial) domain, the I (intermediate) domain, and the A (apical) domain, reflecting their respective spatial positions in the GroEL-GroES chaperone complex.