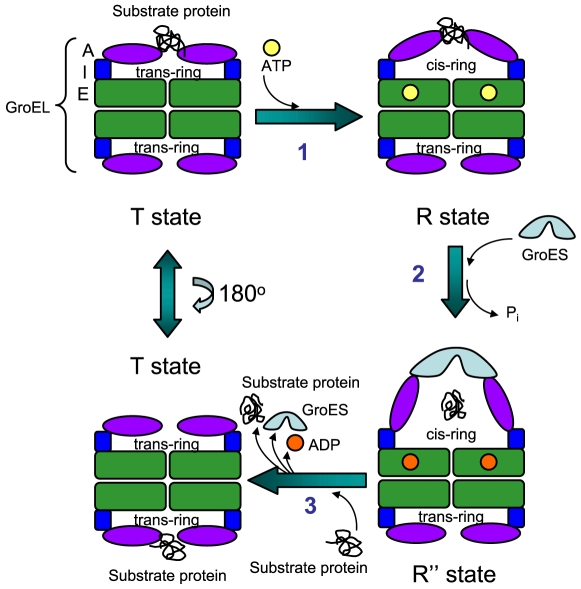
Figure 2. Key conformational states of T, R, and R″ in the simplified allosteric signaling cycle.
Each of the 14 identical GroEL chains contains the E domain (equatorial domain, shown in green), I domain (intermediate, blue), and A domain (apical, purple). In step 1 of this illustration of simplified cycle, the A domains in the T state conformation of the GroEL subunits bind a misfolded or unfolded substrate protein. After step 1, GroEL reaches the R state conformation upon binding of 7 ATP molecules on the E domains. In step 2, the A-domain of the ATP-bound form of GroEL binds to GroES, and the substrate protein falls into the central cavity of the chaperone complex. At the same time, ATPs are hydrolyzed to ADP molecules, and the conformation of GroEL-GroES changes to the R″ state. In step 3, a new unfolded protein ligand binds to the other symmetrically related ring of GroEL. Once bound, the GroES, and the now re-folded substrate protein, and 7 ADP molecules are released. The conformation of the GroEL switches back to the T state.