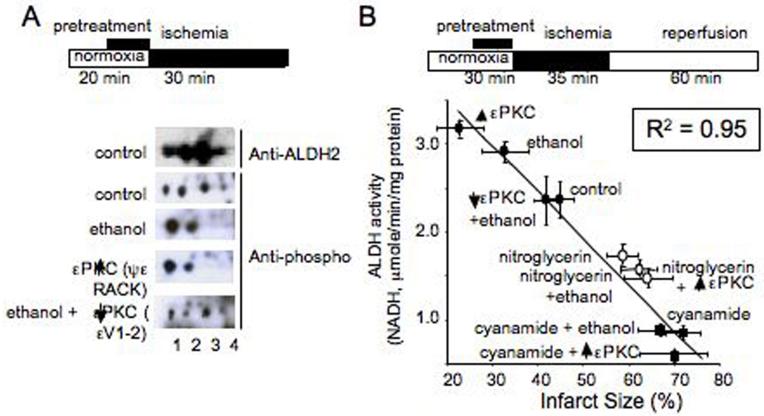
Figure 1. Ethanol and εPKC activation induce phosphorylation of mitochondrial ALDH2.
(A). Homogenates of hearts subjected to ischemia ex vivo were separated by IEF/SDS 2-D gel and probed with a mixture of phospho-serine/threonine antibodies. Using a Langendorff apparatus, hearts were perfused with oxygenated Kreb-Henseleit buffer alone as control, with 50mM ethanol for 10 minutes, with 1μM εPKC agonist (ψεRACK) for 10 minutes (C), or with 1μM εPKC antagonist (εV1-2) for 5 minutes followed by 10 minutes of perfusion together with 50mM ethanol. The hearts were then subjected to a 30 minute period of no-flow ischemia before homogenization. Treatment with ethanol and ψεRACK induced a leftward shift of ALDH2 as compared to control, which was blocked with εV1-2 treatment. Blots were probed with anti-ALDH2 or anti-phospho Ser and Thr (5). (B). ALDH2 activity correlates with cardiac protection from ischemic injury (B). Measurements of ALDH activities in normoxic and ischemic hearts treated with ethanol (EtOH; 50mM), εPKC agonist (ψεRACK) or εPKC antagonist (εV1-2) in the presence of ethanol using the Langendorf apparatus (5). Ischemic hearts were also treated with the ALDH2 inhibitor, cyanamide (CYA) in the presence or absence of ethanol, εPKC agonist and antagonist, and the ALDH2 desensitizer, nitroglycerin (GTN). Shown is ALDH2 activity (μmoles of NADH/min/mg protein) as a function of infarct size, measured by TTC staining from corresponding heart samples derived from the same studies as in Table 1. Linear regression yielded a high inverse correlation of R2 = 0.95.