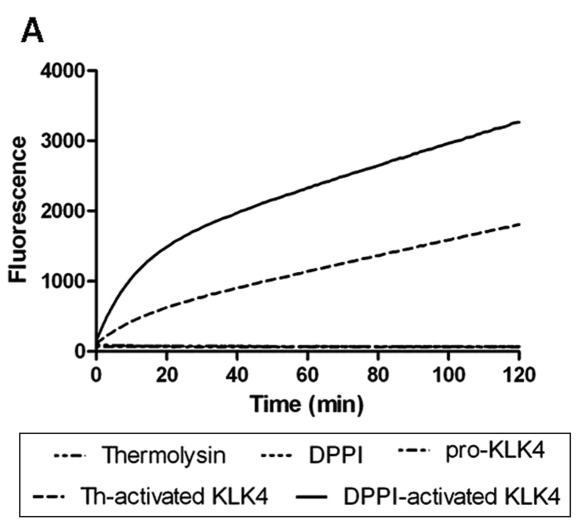
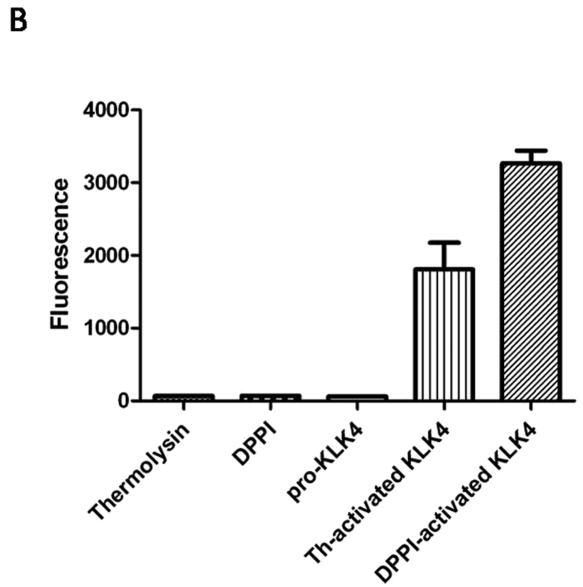
Figure 3.
DPPI activates pro-KLK4 in vitro. (A) We measured hydrolysis of the KLK4 specific substrate (Boc-Val-Pro-Arg-AMC) by monitoring fluorescence every 1 min over 2 hrs with 380-nm excitation and 460-nm emission wavelengths. The fluorescent substrate was incubated with thermolysin, DPPI, pro-KLK4, thermolysin-activated KLK4 (th-activated KLK4), and DPPI-activated KLK4. (B) Fluorescence measurement with the same enzymes after two-hour incubation of the Boc-Val-Pro-Arg-AMC substrate (n = 4). Incubation with thermolysin, DPPI, or pro-KLK4 did not result in substrate cleavage. Thermolysin-activated KLK4 (th-activated KLK4) and DPPI-activated KLK4 both cleaved the substrate, as indicated by the fluorescence. Data are presented as mean ± SEM.