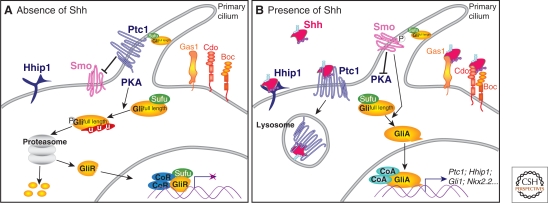
Figure 3.
SHH signal transduction. (A) In the absence of SHH, the receptor Ptc1 represses the activity of the transmembrane protein Smo and the translocation of Smo to the primary cilium of the cell. In these cells, protein kinase A (PKA) promotes the proteasome-dependent partial processing or complete degradation of the Gli transcription factors (Gli2 and Gli3). The truncated forms of these proteins (GliR) translocate to the nucleus and repress the transcription of SHH signaling targets. SuFu maintains any remaining full-length Gli proteins in an inactive state. (B) The binding of SHH to Ptc1 releases repression of Smo, allowing its translocation into the cell's cilium. The activation of Smo inhibits the proteolytic processing of Gli proteins and culminates in activated Gli proteins (GliA) translocating to the nucleus to activate target gene expression. At the cell surface, in addition to Ptc1, the SHH-binding membrane protein Hhip1 binds SHH and inhibits signaling. Conversely, the SHH-binding proteins Gas1, Cdo, and Boc enhance the response of cells to the morphogen.