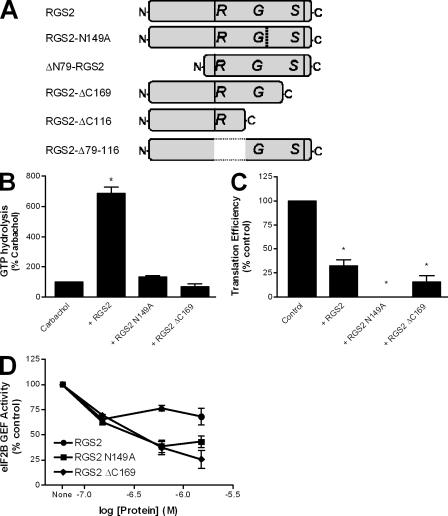
Figure 3.
RGS2-mediated inhibition of protein synthesis is independent of its RGS domain function. (A) Schematic of the purified RGS2 proteins used throughout the study. (B and C) The effects of full-length RGS2, RGS2-N149A, and RGS2-ΔC169 (4 µM) were examined on steady-state, agonist-stimulated GTPase activity of M1 muscarinic receptor–activated Gα11 (B) or synthesis of the reference luciferase protein in a reticulocyte-based in vitro translation assay (C) as described in Materials and methods. The data are presented as mean ± SEM of three independent experiments performed in triplicate. *, P < 0.05 versus control (one-sample t test). (D) The ability of 150 nM purified eIF2B to promote dissociation of [3H]GDP from purified eIF2 was examined as described in Materials and methods. This activity was assessed in the absence and presence of three concentrations of RGS2, RGS2N149A, and RGS2ΔC169. The data are presented as mean ± SEM of four independent experiments performed in duplicate.