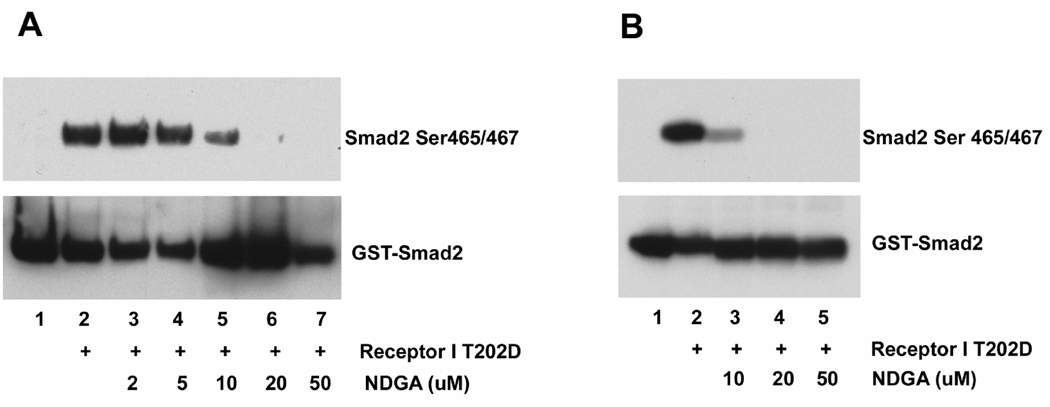
Fig. 5.
NDGA inhibits Smad2 phosphorylation mediated by TGF β type I receptor in vitro. A. Cell lysates from TGF β type I receptor T202D (receptor I T202D) transfected cells were incubated with purified GST-Smad2 fusion protein, and the phosphorylated Smad2 was analyzed by in vitro pull-down followed by immunoblotting. Immunoblotting with anti-GST were used as a pull-down and loading control. Smad2 phosphorylation was observed in reactions using lysates from transfected cells but not in reactions using lysates from control cells. NDGA inhibited Smad2 phosphorylation in a dose-dependent manner. B. Immunoprecipitated TGF β type I receptor T202D (receptor I T202D) were incubated with purified GST-Smad2, and the phosphorylated Smad2 was analyzed by immunoblotting. Immunoblotting with anti-GST was used as a loading control. Immunoprecipitated receptor showed kinase activity against Smad2 while the control immunoprecipitation showed no activity. NDGA inhibited Smad2 phosphorylation in a dose-dependent manner.