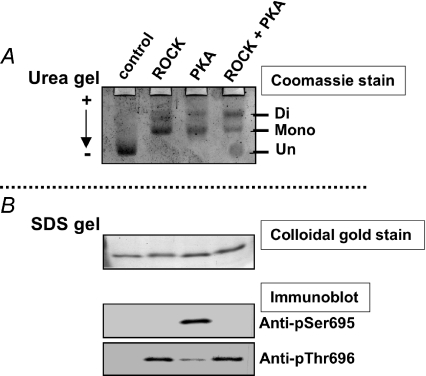
Figure 3. Specificity of anti-phospho[Ser695] and [Thr696] antibodies.
A MYPT1 fragment (658–714) was phosphorylated with ROCK alone, PKA alone, or a mixture of ROCK and PKA. Twenty nanograms of phosphorylated or unphosphorylated fragments were applied onto a urea-lutidine gel (A; Kitazawa et al. 1999) to separate mono- and diphosphorylated bands from un-phosphorylated bands. The gel was stained with Coomassie Brilliant Blue (A). The relative intensity of monophosphorylated band in A was 2, 61, 62 and 38%, and the diphosphorylated band intensity was 0, 39, 38 and 62% for control, ROCK, PKA and ROCK+PKA, respectively. The same amount of the samples was also applied onto a SDS-polyacrylamide gel (B) to detect total and phosphorylated amounts of fragments using, respectively, colloidal gold staining (upper panel in B) and Western blots with phospho- and site-specific antibodies (two lower panels). Arrow in A indicates the direction of current in the urea-lutidine gel. The anti-phospho[Ser695] antibody did not detect diphosphorylated fragments treated with ROCK+PKA, suggesting that this antibody recognizes only mono-phosphorylated Ser695 (Wooldridge et al. 2004). The anti-phospho[Thr696] antibody identified both mono- and di-phosphorylated Thr696.