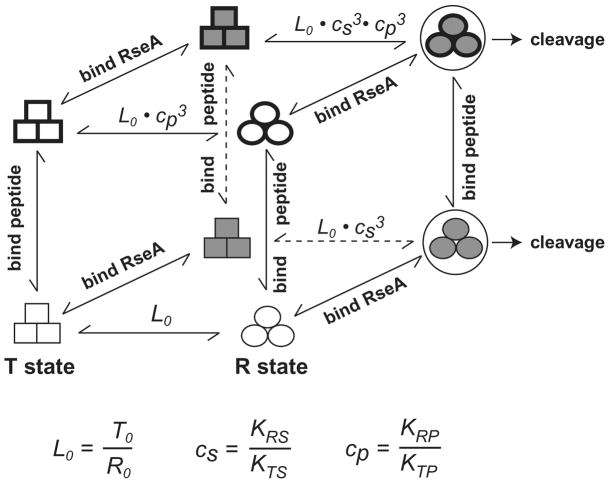
Figure 5.
Allosteric relationships.
Equilibria between relaxed DegS trimers (circles) and tense DegS trimers (squares) are perturbed by the binding of OMP peptides and RseA substrate. For simplicity, partially liganded states are not shown. Species that comprise the top face of the cube have three OMP peptides bound; species that comprise the back face of the cube have three RseA substrates bound. The front face of the cube consists of substrate-free trimers. The bottom face of the cube consists of peptide-free trimers. Horizontal arrows indicate conformational equilibria. Vertical arrows signify reactions in which three OMP-like peptides associate or dissociate from DegS. Arrows at an angle indicate reactions in which three molecules of the RseA substrate associate or dissociate from DegS. Cleavage of RseA only occurs from substrate-bound relaxed DegS.