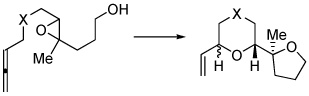
Table 1.
Scope of the Allene Initiated Cascade Cyclization
| substrate | product(s) | yielda | selectivity | |
|---|---|---|---|---|
 |
(trans cis)b | |||
| 1 | X = (PhO2S)2C | 2a,b | 65% | 2.2:1 |
| 3 | X = O | 4a,b | 60% | 1:8 |
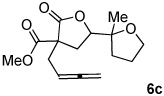
| 5 | X = (MeO2C)2C | 6a,b | 65%c | 1:8 |
 |
||||
 |
||||
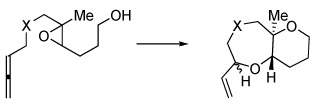
| 7 | X = (PhO2S)2C | 8a,b | 35% | 7:1 |
| 9 | X = NTs | 10a,b | 59%d | 4:1 |
| 11 | X = (MeO2C)2C | |||
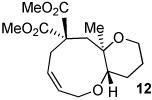 |
40% | --e | ||
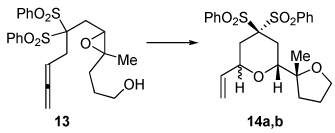 |
45% | 2.5:1 | ||
General conditions: 5 mol % (PhO)3PAuCl / 5 mol % AgOTf, CH2Cl2, rt, 15–45 min.
Isolated yields after silica gel chromatography.
Relative stereochemistry of ethereal α and α’ stereocenters in the vinyl-bearing oxacycle.
~10% of 6c also generated.
<10% of the 9-endo product also formed.
Only one isomer detected by 1H, 13C NMR.
