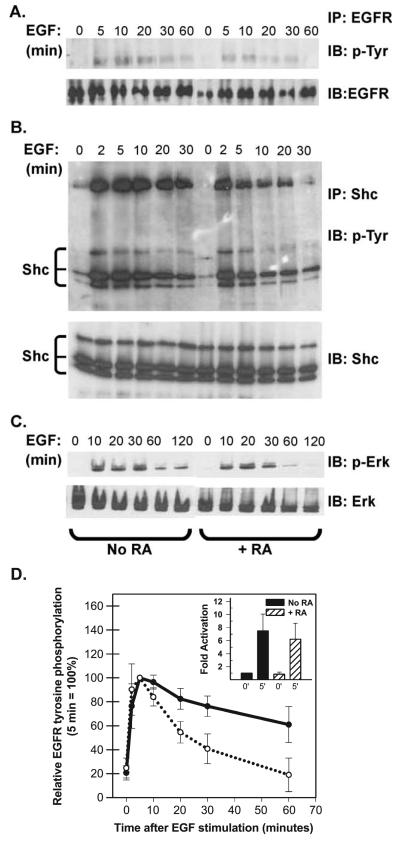
Fig. 3.
RA reduces the duration of EGF signaling. (A) T-47D cells were serum starved in the absence or presence of 10−7 M RA for 36 h and then stimulated with 40 ng/ml EGF. At the times indicated: (A) Cells were harvested and EGF receptor (EGFR) was recovered by immunoprecipitation (IP) and resolved by SDS-PAGE. After transfer to nitrocellulose, blots were sequentially probed with an anti-phosphotyrosine antibody (4G10, IB: pTyr) and then, after stripping, with an anti-EGFR antibody (IB: EGFR). Only the region from the gel corresponding to the approximately 175-kDa standard is shown. (B) Cells were immunoprecipitated with a polyclonal antibody directed against the adapter protein Shc. Immunoprecipitated proteins were resolved by SDS-PAGE, transferred to nitrocellulose and the filters were probed sequentially with the monoclonal 4G10 antibody (IP: Shc, IB: p-Try) and a monoclonal anti-Shc antibody (IB: Shc). The positions of Shc isoforms and the co-immunoprecipitated EGFR (confirmed by additional immunoblotting, not shown) are indicated. (C) Or, Erk 1/2 activation was assessed by sequential immunoblotting of whole cell extracts (30 Ag/lane) with anti-phospho-Erk antibodies (IB: p-Erk) and anti-Erk antibodies (IB: Erk). (D) EGFR tyrosine phosphorylation was assessed as described above and signal strength was quantified by densitometry. The data plotted in panel D show the degree of EGFR staining with anti-phosphotyrosine antibodies after various times of EGF stimulation (averaged from five independent experiments ± SEM). The inset shows that the average fold activation at 0 and 5 min did not differ between control (=1) and RA-pretreated cells.