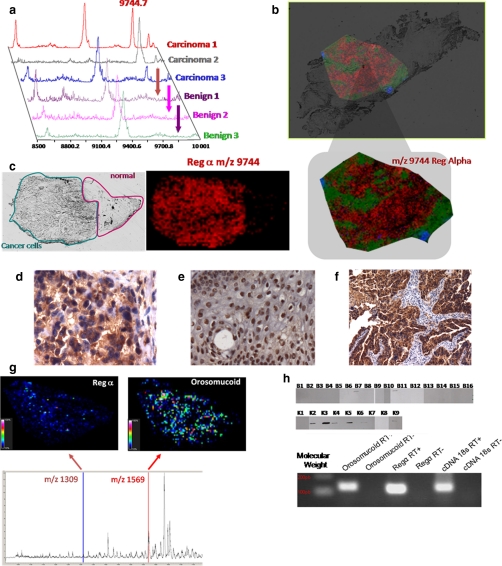
Fig. 6.
Validation of C-terminal fragment of the immunoproteasome REG-α and orosomucoid as ovarian biomarkers. a, MALDI-MS profiles of three ovarian carcinomas versus benign tumor samples. b, MALDI-MSI molecular image of REG-alpha fragment (m/z 9744) at a resolution of 50 μm from an ovarian carcinoma tissue section. c, optical image of the tissue section with the region of interest defined (cancerous versus healthy part) and MALDI-MSI of the REG-α fragment showing its presence exclusively in the cancer part. d–f, immunocytochemical data obtained after the antigen retrieval technique and H&E coloration with the anti-C-terminal REG-α antibody: d, cytoplasm localization of the anti-C-terminal REG-α labeling in ovary carcinoma; e, nucleus localization of the anti-C-terminal REG-α labeling in ovary benign tumor; f, epithelial cells labeled with the anti-C-terminal REG-α in ovarian carcinoma. g, specific MALDI imaging analysis using the Tag-mass concept with anti-C-terminal REG-α and an anti-human IgG tag (reporter m/z 1309) and anti-orosomucoid and anti-human monoclonal antibody (reporter m/z 1569). h, top, Western blot analyses with the anti-C-terminal REG-α (immunoproteasome 11 S) of the 16 benign tumors and nine carcinomas (33); bottom, quantitative PCR validation of REG-α and orosomucoid from the SKVO3 ovarian cancer epithelial cell line.